Received: July , 2014
Fluorine Notes, 2014, 96, 3-4
THE SYNTHESIS AND PROPERTIES OF 1-POLYFLUOROALKOXY-1-PROPENOXYETHANES
A. I. Rakhimov1,2, O. N. Kutyga1
1Volgograd State Technical University, 400131, Russia, Volgograd, Lenin prospect, 28
e-mail:
organic@vstu.ru
2Institute of Chemical problems of Ecology ANS RF, 400066, Volgograd PO box 127,
e-mail:
rakhimov@sprint-v.com.ru
Abstract: 1-Polyfluoroalkoxi-1-propenoxyethanes are obtained using nucleophilic substitution of chlorine in α-chloropolyfluoroalkyl ethers according to their reaction, with allyl alcohol; the polymerization of allyl ethers has been carried out and hydrophobic properties of polymers obtained have been studied.
Keywords: 1-polyfluoroalkoxi-1-chloroethanes, α-chloropolyfluoroalkyl ether, 1-polyfluoroalkoxi-1-propenoxyethane, polymerization, hydrophobization.
Earlier we have considered [1-5] the synthesis of α-chloroethers and the reactions of nucleophilic substitution for different functional groups.
Perfluorinated ketones treated by metals fluorides were offered to obtain polyfluorinated compounds with allylic group, and allylhalogenide was used as an impact compound for formed pefluorinated [6].
We have offered a more simple method of obtaining of allyl ethers based on polyfluorinated telomers obtained by polymerization of tetrafluoroethylene with methanol [3]. 2-Chloroethers based on polyfluorinated telomeric alcohols are obtained according to method developed [1, 2]:
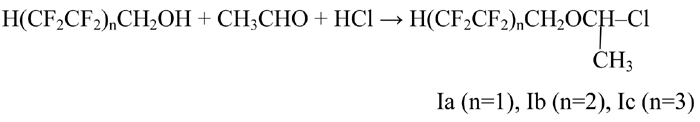
α-Chloropolyfluorinated ethers were treated with allyl alcohol in organic solvent (dried hexane), continuously removing isolating chlorine hydride using inert gas (nitrogen). The reaction was carried out at temperature within the range of 30-35 °C for tetrafluoropropanol and at temperature within the range of 40-45 °C for more long-chain polyfluorinated α-chloroethers:
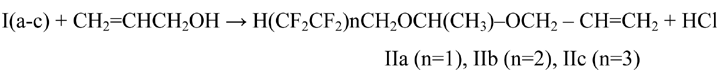
The yield of 1-polyfluoroalcoxy-1-propenoxyethanes IIa, IIb, IIc are decreasing while the length of perfluorinated chain increases in the initial I(a-c) α-chloroether: 75,3 % (IIa), 70,3 % (IIb) and 68,5 % (IIc) (Table 1). In the ethers’ IR-spectra the initial compounds are not found, the variations of C–O and C–F bonds are observed. In H1NMR-spectra the triplet of triplets of HќCF2-group proton is observed (δ 3,97 ppm), we also observe triplets with the center at δ 3,97 ppm, groups –CHќ2O- , doublet of protons of–CHќO- group with δ 3,95 ppm and δ 1,28 ppm, singlet with δ 1,80 ppm corresponds to protons of methyl group – CHќ3. The signals of methyl protons of allyl group reveal themselves in a weak field (4, 1 and 4,24 ppm). The polymerization of obtained monomers was initiated using benzoylperoxide, which is widely used as an initiator [3, 4].
The polymerization of monomers was being carried out at 100 °C for 10 hours, (amount of benzoylperoxide – 0,01 g per 5 g of monomer), the polymer was purified by re-sedimentation of hexane from ether with further isolating of solvent in vacuum. The polymer was an oily mass.
The testing of hydrophobic properties of obtained polymers was carried out using samples of glass fibre cloth. At that, the solutions of polymers in chladone 113 of different concentration were used. The samples of cloth were dipped into polymer’s solution, afterwards they were air dried for 10-20 min and thermally treated at 120 °C during 15 min.
As you can see from the Table 2 the cloth processed with 1-polyfluoroalcoxy-1-propenoxyethanes with the longest (n=3) chain has got the best hydrophobic characteristics and it exceeds the hydrophobicity of the ether described in the patent [7].
Thus we found that 1-polyfluoroalcoxy-1-propenoxyethanes are the available class of new hydrophobizing agents.
Experimental Part
Fluorine containing allyl ethers were synthesized by the following common method.
To the 0,05 mole of α-chloroalkylpolyfluoroalkyl ether into the 50 ml of dry hexane a 0,05 mole of allyl alcohol in 30 ml of dry hexane is added (temperature is 0°C). The isolating hydrogen chloride is removed by the input of dry nitrogen gradually increasing the temperature up to 30-35 °C and mixing for 3-4 hours. After removing of solvent the residue is being distilled into vacuum. The yield and constant of allyl ethers are listed in Table 1.
Table 1. 1-Polyfluoroalkoxi-1-propenoxyethanes, H(CF2CF2)nCH2OCH(CH3)CH2=CH–CH2.
Table 2. Hydrophobic Properties of Glass Fibre Cloth Treated With Polymer Solution Based On Allyl Ethers With Polyfluoroalkyl Groups.
|
Hydrophobizing Compound H(CF2CF2)nCH2OCH(CH3)CH2=CH–CH2 |
Solution Concentration, % |
Water absorption |
|
n = 1 |
1 2 |
52,0 52,3 |
|
n = 2 |
1 2 |
49,2 49,0 |
|
n = 3 |
1 2 |
48,5 47,5 |
|
unhydrophobized cloth |
- |
66,1 |
References
- Rahimov A.I., Kutyga O.N., Bakshaeva A.A. Sintezα-hlorpoliftoralkilovyh ehfirov i acetalej na ih osnove. ZhOH, 2011, t. 81, vyp. 3, s. 463-466.
- Rahimov A.I., Kutyga O.N., Bakshaeva A.A. Sintez di(1,1,3- trigidroperftorpropoksi)metana i ehtana.ZhOH, 2009, t. 79, vyp. 11,s. 1926-1927.
- Rahimov A.I. Khimiya i tekhnologiya ftororganicheskih soedinenij. M. Khimiya: 1986.271 s.
- Rahimov A.I. Khimiya i tekhnologiya organicheskih peroksidnyh soedinenij. M.: Khimiya, 1979. 389 s.
- Rahimov A.I., Tihonova E.G.α-Chlorehfiryα-oksialkilperekisej. ZhOH, 1977, t. 8, p. 318-324.
- Pittman A.G. Jharp O.L., Ludwig B.A. J. Polym.: Sci., 1968, AI, G, 741.
- Pittman A.G, William L. Wasley US 3382222 A Fluorinated allyl ethers and use there of, 1965.
Recommended for publication by Prof. A. I. Rakhimov
Fluorine Notes, 2014, 96, 3-4
