Received: May , 2014
Fluorine Notes, 2014, 95, 5-6
STUDY ON THE SURFACE STRUCTURE OF CARBOXYMETHYL CELLULOSE WITH OCTAFLUOROPENTYL FRAGMENT
A.I.Rahimov1, N.A.Rahimova2, E.V. Petrosyan 2
1Institute of Chemical problems of Ecology ANS RF, 400066, Volgograd PO box 127
2Volgograd State Technical University, 400131, Russia, Volgograd, Lenin prospect, 28
e-mail:
organic@vstu.ru
Abstract: The modification of carboxymethyl cellulose with octafluoropentyl fragment is shown to result in the formation of a polymer with improved hydrophobic properties while keeping high water solvency.
Key words: carboxymethyl cellulose, octafluoropentyl chlorosulfite, hydrophobic properties.
Introduction
One application of polyfluorinated alcohols (wastes from tetrafluoroethylene/methanol telomerization [1]) is polyfluoroalkylation of polysaccharides, particularly, that of carboxymethyl cellulose, widely used in various industrial sectors.
Modification of carboxymethyl cellulose with octafluoropentyl fragments [2-8] allows considerable alteration of the polymer physicochemical properties and providing unique characteristics useful, e.g., in drill fluids employed under strict conditions.
The study objective was to investigate the impact of octafluoropentyl fragment on the surface structure of carboxymethyl cellulose.
Experimental
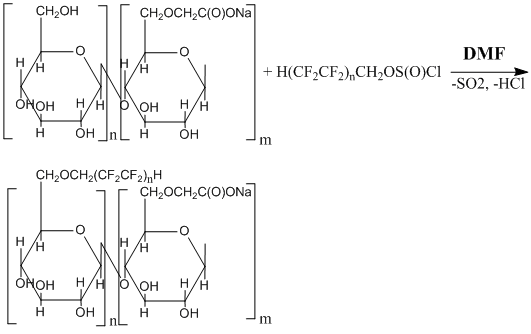
The introduction of octafluoropentyl groups into macromolecules of carboxymethyl cellulose was conducted through directed modification of hydroxyls by octafluoropentylchlorosulfite. This modification of the polymer surface occurred in chloroform environment, and dimethylformamide was used for catalyst.
Morphology
Polymer surface structure was investigated with the help of a scanning electronic microscope Versa 3D DualBeam.
Contact angles
Contact angles were measured with the help of OCA 15 (DataPhysics Instruments GmbH, Germany)
IR spectral analysis
Powder samples of carboxymethyl cellulose and polyfluoroalkylated carboxymethyl cellulose (F content was 6.67%) were studied with the help of IR Fourier spectrometer «Nicolet-6700» (Thermo Electron, USA).
Results and discussion
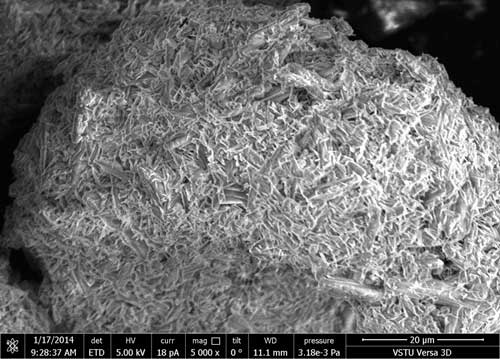
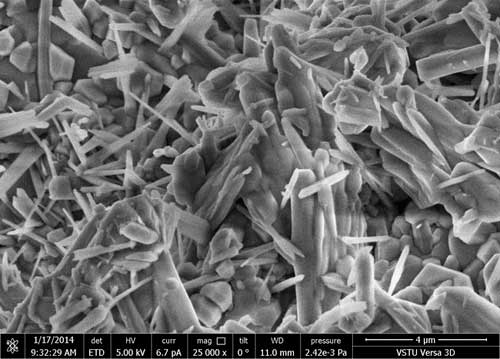
Figs. 1 (a), (b), (c) represent micrographs of initial carboxymethyl cellulose at various magnifications. In Fig.1 one can see that polymer consists of similarly structured filaments.
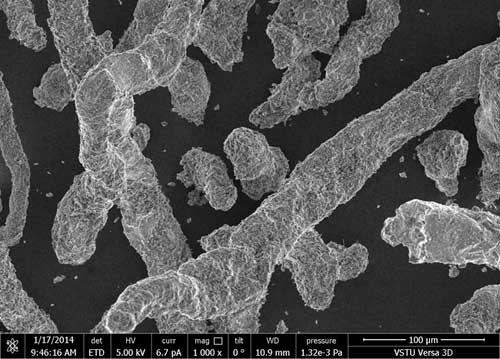
Figs, 2 (a), (b), (c) represent polyfluoroalkylated carboxymethyl cellulose at various magnifications (1000-25000 times). The polymer structure after the treatment becomes uniformly spherical.
 (a)
(a)
 (b)
(b)
 (c)
(c)
Figure 1. Micrographs of carboxymethyl cellulose at various magnifications: 1000 (a); 5000 (b); 25000 (c)
 (a)
(a)
 (b)
(b)
 (c)
(c)
Figure 2. Micrographs of polyfluoroalkylated carboxymethyl cellulose at various magnifications: 1000 (a); 10000 (b); 25000 (c).
From the comparison of surface micrographs of initial carboxymethyl cellulose and polyfluoroalkylated carboxymethyl cellulose one may see considerable alteration of the solid surface characteristics. The surface structure of carboxymethyl cellulose with octafluoropentyl fragment is more uniform and smooth, and the presence of octafluoropentyl fragment contributes to the surface hydrophobic properties.
The contact angle of a film produced of 2% aqueous solution of initial carboxymethyl cellulose is 6.07° (figure 3 (a)). After treatment of carboxymethyl cellulose with octafluoropentyl fragment, the contact angle of the film produced of 2% aqueous solution of polyfluoroalkylated carboxymethyl cellulose is 11.02° (figure 3 (b)). Those measurement results evidence growth of the material hydrophobic properties, while keeping good water solubility.
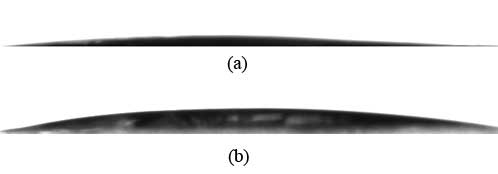
Figure 3. Edge water contact angles of a film made of carboxymethyl cellulose (a) and polyfluoroalkylated carboxymethyl cellulose (b).
IR Fourier spectra of initial and polyfluoroalkylated carboxymethyl cellulose are present in Figures 4 (a) and (b). One of the main distinctions due to the modification is the absence of absorption peaks at 1284 cm-1 and 1310 cm-1 , thanks to the alteration of the surface structure, and apparition of a number of new peaks at 681 cm-1, 698 cm-1, 759 cm-1, 833 cm-1, 1139 cm-1 and 1720 cm-1, assigned to octafluoropentyl groups (Table 1).
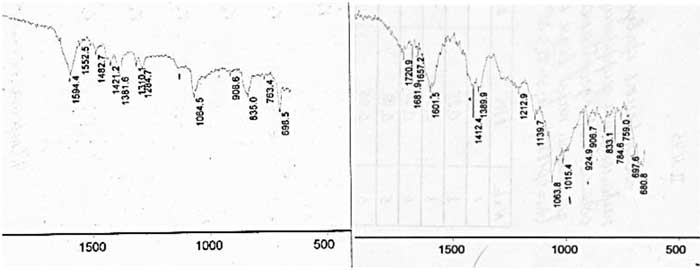 (a,b)
(a,b)
Figure 4. IR Fourier spectra of carboxymethyl cellulose (a) and polyfluoroalkylated carboxymethyl cellulose (b).
Table 1. IR absorption spectra (peaks) of carboxymethyl cellulose and polyfluoroalkylated carboxymethyl cellulose.
|
Group |
Absorption bands in IR spectra, cm-1 |
|
|
carboxymethyl cellulose |
polyfluoroalkylated carboxymethyl cellulose |
|
|
HO, COOH |
1284, 1310,1381, 1421, 3500-3600 |
1390, 1412, |
|
-COONa |
1594 |
1601 |
|
Cyclo- CH2-O-CH2- |
1064 |
1064 |
|
-(CF2)4- |
- |
681, 698, 759, 833, 1139, 1720 |
Conclusion
By the means of scanning electronic microscopy, measurements of edge contact angle, and Fourier IR spectrometry the surface structure of carboxymethyl cellulose is investigated after the introduction of octafluoropentyl fragments. It is found that a layer thus formed on the film surface contains octafluoropentyl groups, providing hydrophobic properties to the polymer.
References
2. Rahimov, A.I.The distinctive features of carboxymethylcellulose sodium salt surface polyfluoroalkyl-modification// Fluorine notes / A. I. Rahimov, O. V. Vostrikova, E. V. Petrosyan. - 2013. -T.89., v. 4, p. 1-2
3. Rahimov, A.I. Novyj metod sinteza poliftorirovannyh prostyh ehfirov // Zhurnal organicheskoj khimii / A. I. Rahimov, A. V. Nalesnaya, O. V. Vostrikova. - 2004. - T. 74. , v. 4. - p. 1121.
4. Rahimov, A. I. Reakciya poliftoralkilhlorsul'fitov s benzilovymi spirtami // Zhurnal obshchej khimii / A. I. Rahimov, R. V. Fisechko. - 2007. - T. 77, v. 10. - p. 1750-1751.
5. Rahimov, A.I. Novyj metod sinteza poliftoralkilciklogeksilovyh ehfirov // Zhurnal obshchej khimii / A. I. Rahimov, R. V. Fisechko. - 2008. - T. 78, p. 2. - p.338.
6. Rahimov, A.I. Osobennosti kataliza reakcii poliftoralkil- chlorsul'fitov s predel'nymi odnoatomnymi spirtami // Zhurnal obshchej khimii / A. I. Rahimov, A. V. Nalesnaya, R. V. Fisechko. - 2008. - T. 78, v. 11. - p. 1842-1848.
7. Patent RU 2346926, MPK C 07 C43/192, C 07 C43/12, C 07 C43/225, C 07C 43/174. Sposob polucheniya prostyh poliftoralkilovyh ehfirov / A.I. Rahimov, A.V. Nalesnaya, R.V. Fisechko; GOU VPO VolgGTU. - 2009.
8. Rahimov, A.I. Sintez poliftoralkilhlorsul'fitov i novye reakcii s ih uchastiem // Zhurnal obshchej khimii - 2010. - T. 80, v. 8. - C. 1622-1641.
Recommended for publication by Prof. A.I. Rahimov
Fluorine Notes, 2014, 95, 5-6
