Received: July , 2014
Fluorine Notes, 2014, 95, 3-4
Synthesis fluorocontaining derivatives of pyrazolo[3,4-d]pyrimidines. Message 1. Synthesis of fluorocontaining 1-phenyl-1H-pyrazolo[3,4-d] [1,3]oxazines and fluorobenzamides of 5-(fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid
M.E.Zhidkov, A.V.Kutkin, A.F.Eleev
State Research Institute of Organic Chemistry & Technology 23, Shosse Entuziastov, 111024 Moscow,
Russian Federation
e-mail: dir@gosniiokht.ru
Abstract: Ethyl ester of 5-amino-1-phenyl—1H-pyrazol-4-carboxylic acid was involved into the reaction with various chloroanhydrides and received appropriate ethyl esters of 5-substituted 1-phenyl-1H-pyrazole-4-carboxylic acid, which in turn were converted into the corresponding acids. Of these acids in the reactions with acetic anhydride were received new pyrazolo[3,4-d] [1,3]oxazines. On their basis synthesized previously not known benzamide pyrazole-4-carboxylic acid.
Keywords: Ethyl ester ethoxymethylenemalononitrile acid, hexazinone, pyrazole, pyrimidine, thin layer chromatography, 1H NMR-spectra.
The derivatives of pyrazolo[3,4-d]pyrimidines with the common formula:
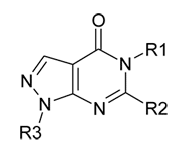
now are represented with a vast number of examples of biologically active substances with antimicrobial [1,2], antivirus [3,4], antiallergic [5], antigipertenzive [6-8], anticancer [9], anti-inflammatory [10] and analgesic activity [11]. General characteristic of biological activity pyrazolo[3,4-d]pyrimidines are presented in the review [12].
The synthesis close in structure to this article is the connection [13]. In this paper we propose a fundamentally new method of production, which is based on the scheme of chemical transformations, including a synthesis of relevant pyrazolo[3,4-d][1,3]oxazinones and on their basis the synthesis of benzamide pyrazole-4-carboxylic acids.
As is known, the introduction of even one of the fluorine atom in the molecule of biologically active substances can lead to a significant change of this or that biological activity. With this purpose for the subsequent study of biological activity of fluorinated analogues derivatives pyrazolo[3,4-d]pyrimidines in this study assessed the possibility of the synthesis of previously unknown analogues of benzamide pyrazole-4-carboxylic acid, containing fluorine atom as a substituent in the 2nd, 3rd or 4th position of benzoyl fragment. Synthesis of target compounds comprises several stages. In the first stage was carried out the synthesis of ethyl ether ethoxymethylenemalononitrile acid according to the scheme:

Further, the obtained ether (1) was involved into the reaction with phenyl-hydrazine with preparation of phenyl containing pyrazole according to the sheme:
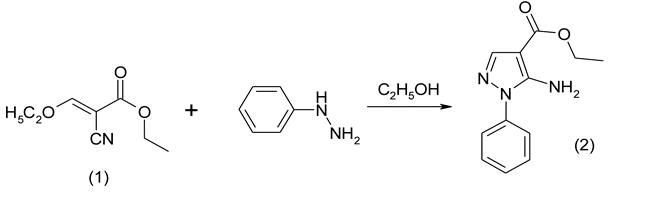
Synthesized phenylsubstituted pyrazole (2) is the first precursor in the synthesis of target compounds. In particular, using 3-fluorobenzoyl chloride was synthesized, according to the developed method of acylation in the anhydrous boiling 1,4-dioxane without the acceptor of hydrogen chloride, ethyl ester 5-(3-fluorobenzoylamino)-1-phenyl-1H-pyrazole-4-carboxylic acid (3a) according to the scheme:

Using a similar scheme with the use of chloroanhydrides of the appropriate acids were synthesized following ethyl esters: 5-(2'-fluorobenzoylamino)- (3b), 5'-(4-fluorobenzoylamino)- (3c), 5-(4'-chlorobenzoylamino)- (3d) and 5(2'-furoylamino)- (3e) 1-phenyl-1H-pyrazole-4-carboxylic acids. These esters in the reaction with sodium hydroxide according to the developed by us method of hydrolysis with water solution of sodium hydroxide at a temperature of 50 В°C later were converted into the corresponding carboxylic acid scheme as shown at the example of a 5-(3'-fluorobenzoylamino)-1-phenyl-1H-pyrazole-4-carboxylic acid (4a):

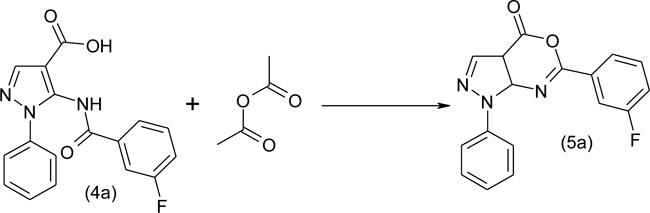
Synthesized 5-(3'-fluorobenzoylamino)- (4a), 5-(2'-fluorobenzoylamino) (4b), 5-(4'-fluorobenzoylamino)- (4c), 5-(4'-chlorobenzoylamino)- (4d) and 5(2'-furoylamino)- (4e) 1-phenyl-1H-pyrazole-4-carboxylic acids, in turn, in the reaction with the acetic anhydride were converted to the corresponding 6-aryl-substituted 1-phenyl-1H-pyrazolo[3,4-d][1,3]oxazin-4-ones. The scheme provides the preparation of the 6-(3'-fluorobenzoylamino)-1-phenyl-1H-pyrazolo[3,4-d][1,3]oxazin-4-one (5a):
Thus obtained oxazine-4-ones were involved in the reaction with various aromatic amines with the formation of the corresponding 5-(fluorobenzoylamino)-substituted arylamides of 1-phenyl-1H-pyrazole-4-carboxylic acid. Below is the scheme of preparation of 4"-fluorophenyl amide of 5-(3'-fluoro-benzoylamino)-1-phenyl-1H-pyrazole-4-carboxylic acid in the reaction of 6-(3'-fluorobenzoyl-amino)-1-phenyl-1H-pyrazolo[3,4-d][1,3]oxazin-4-one with 4-fluoroaniline:
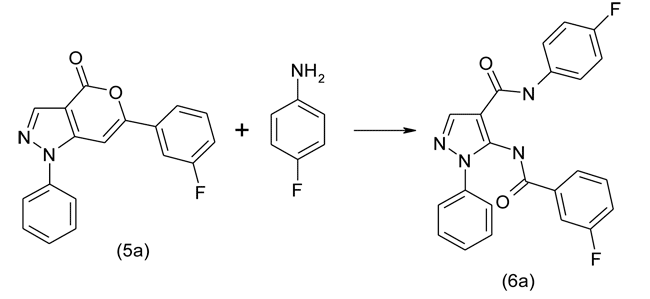
By the similar scheme were synthesized: 1"-naphthylamide of 5-(2'-fluorobenzoylamino)-1-phenyl-1H-pyrazole-4-carboxylic acid (6b), 4"-fluorophenylamide of 5-(4'-chlorobenzoylamino)-1-phenyl-1H-pyrazole-4-carboxylic acid (6c), 2"-(ethoxycarbonyl)phenylamide of 5-(4'-fluorobenzoylamino)-1-phenyl-1H-pyrazole-4-carboxylic acid (6d), 4"-chlorophenylamide of 5-(3'-fluorobenzoylamino)-1-phenyl-1H-pyrazole-4-carboxylic acid (6e), 2",4"-dimethoxyphenylamide of 5-(2'-fluorobenzoylamido)-1-phenyl-1H-pyrazole-4-carboxylic acid (6f).
Experimental
1H NMR spectra (external reference TMS) were obtained in DMSO-d6 on the device Bruker AM-400 (400 MHz), melting temperatures of the substances were defined on the device Mettler FP5. Monitoring the progress of the reaction and individuality of the obtained compounds was carried out by TLC on plates Silufol UV-254 in the system of toluene-acetone 4:1.
Ethoxymethylenecyanoacetic acid ethyl ester (1) In a three-neck flask on 500 ml, equipped with a stirrer, thermometer, and a coloumn with condenser were placed 111,2 g (124,75 ml; 0,75 mol) triethylortoformate, 56,6 g (53,2 ml, 0,5 mol) cyanoacetic acid ethyl ester and 102,1 g (94,5 ml, 1,0 mol) acetic anhydride. The reaction mass was stirred at the temperature 140В°C for 1 hour and then at the temperature 150В°C for another 1 hour. Ethyl acetate was removed during this procedure. The obtained product was distilled under reduced pressure, afforded 69,3 g (82,0%) of ethoxymethylenecyanoacetic acid ethyl ester with mp. 50-52 В°C and bp..173-175 В°C under 15 mm.Hg. Rf 0,56 (toluene-acetone 4:1). NMR spectrum 1H (30% in CDCl3), Оґ m.d.: 1,29 (3H, t, CH3), 1,42 (3H, t, CH3), 4,30 (2H, q, CH2), 4,49 (2H, q, CH2), 8,03 (1H, s, CH=). Found, %: C 56,21; H 6,51; N 8,36. C8H11NO3 Calculated, %: C 56,80; H 6,55; N 8,28.
5-Amino-1-phenyl-1H-pyrazolo-4-carboxylic acid ethyl ester (2) In a three-neck flask on 500 ml, equipped with a stirrer, thermometer, and a condenser were placed 69,0 g (0,41 mol) ethoxymethylenecyanoacetic acid ethyl ester and 100 ml of ethyl alcohol. The solution of 44,3 g (0,41 mol) of pheylhydrazine in 100 ml of ethyl alcohol was added dropwise with stirring. The reaction mixture was stirred under reflux for 1 hour and 150 ml of ethyl alcohol were evaporated. The the reaction mixture was cooled on the ice bath and the obtained crystals were filtered, washed with cold ethyl alcohol and dried. Recrystallization from ethyl acetate gave 65,0 g (68,5%) of 5-amino-1-phenyl-1H-pyrazolo-4-carboxylic acid ethyl ester as a pale-yellow crystals with mp 100 В°C. Rf 0,65 (toluene-acetone 4:1). NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 1,39 (3H, t, CH3); 4,25 (2H, q, CH2); 5,39 (1H, s, NH); 7,39 (1H, m, C6H5); 7,50 (4H, m, C6H5); 7,74 (1H, s, CH=). Found, %: C 61,97; H 5,73; N 18,21. C12H13N3O2 Calculated, %: C 62,33; H 5,67; N 18,17.
5-(3-Fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid ethyl ester (3a) In a three-neck flask on 100 ml, equipped with a stirrer, thermometer, and a condenser were placed 23,1 g (0,1 mol) 5-amino-1-phenyl-1H-pyrazolo-4-carboxylic acid ethyl ester, 17,7 g (0,11 mol, 13,1 ml) 3-fluorobenzoyl chloride and 50 ml of absolute 1,4-dioxane. The reaction mixture was stirred under reflux for 10 hours and then cooled. Obtained crystals were filtered, washed with cold ethyl alcohol and dried. Thus was prepared 29,0 g (82%) 5-(3-fluoro-benzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid ethyl ester as a white crystals with mp 112-114В°C. Rf 0,45 (toluene-acetone 4:1). NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 1,30 (3H, t, CH3); 4,24 (2H, q, CH2); 7,15 (2H, m, C6H4F); 7,29 (1H, m, C6H5); 7,54 (1H, t, C6H4F ); 7,81 (1H, s, CH=); 8,14 (2H, m, C6H4F); 11,17 (1H, s, NH). Found, %: C 64,76; H 4,39; N 11,83; F 5,41. C19H16FN3O3 Calculated, %: C 64,58; H 4,56; N 11,89; F 5,38.
According to the similar method with use of various acylating agents were synthesized the following compounds:
- 5-(2-Fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid ethyl ester (3b) with output 75,0% as the white powder with mp.102-105 В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 1,30 (3H, t, CH3); 4,24 (2H, q, CH2); 7,15 (1H, t, C6H4F); 7,29 (1H, m, C6H5); 7,54 (6H, m, C6H5 ); 7,81 (1H, s, CH=); 8,14 (2H, m, C6H4F); 11,17 (1H, s, NH). Found, %: C 64,51; H 4,51; N 11,83; F 5,33. C19H16FN3O3 Calculated, %: C 64,58; H 4,56; N 11,89; F 5,38.
- 5-(4-Fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid ethyl ester (3c) with output 83,0% as the white powder with mp.127-130 В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 1,30 (3H, t, CH3); 4,24 (2H, q, CH2); 7,37 (2H, t, C6H4F); 7,54 (5H, s, C6H5 ); 7,81 (1H, s, CH=); 8,16 (2H, t, C6H4F); 11,17 (1H, s, NH). Found, %: C 64,71; H 4,58; N 11,92; F 5,42. C19H16FN3O3 Calculated, %: C 64,58; H 4,56; N 11,89; F 5,38.
- 5-(4-Chloroobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid ethyl ester (3d) with output 87,0% as the white powder with mp.119-124 В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 1,30 (3H, t, CH3); 4,24 (2H, q, CH2); 7,54 (7H, m, C6H5 ); 7,81 (1H, s, CH=); 7,90 (2H, d, C6H4Cl); 11,17 (1H, s, NH). Found, %: C 61,82; H 4,39; N 11,41; Cl 9,63. C19H16ClN3O3 Calculated, %: C 61,71; H 4,36; N 11,36; Cl 9,59.
- 5-(4-Furoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid ethyl ester (3e) with output 87,0% as the white powder with mp.97-100 В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 1,30 (3H, t, CH3); 4,24 (2H, q, CH2); 6,71 (1H, t, C4H3O); 7,34 (1H, d, C4H3O); 7,54 (5H, d, C6H5); 7,81 (1H, s, CH=); 7,95 (1H, d, C4H3O), 11,17 (1H, s, NH). Found, %: C 62,81; H 4,58; N 12,89. C17H15N3O4 Calculated, %: C 62,76; H 4,65; N 12,92.
5-(3-Fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid (4a) In a three-neck flask on 100 ml, equipped with a stirrer, thermometer, and a condenser were placed 29,0 g (0,082 mol) 5-(3-fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid ethyl ester and the solution of 6,6 g (0,164 mol) sodium hydroxide in 50 ml of water. The reaction mixture was stirred under the temperature 50В°C for 5 hours. Then the reaction mixture was filtered, acidified until pH 2-3 with 10% solution of hydrochloric acid and the obtained precipitate was filtered. The precipitate was carefully washed with water and dried. Thus was prepared 20,3 g (76,3%) 5-(3-fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid as a white crystals with mp.196-199В°C. Rf 0,1 (toluene-acetone 4:1). NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 7,15 (2H, m, C6H4F); 7,29 (1H, m, C6H5); 7,54 (4H, m, C6H5 ); 7,81 (1H, s, CH=); 8,14 (2H, m, C6H4F); 11,17 (1H, s, NH); 11,95 (1H, s, COOH). Found, %: C 62,81; H 3,69; N 12,91; F 5,78. C17H12FN3O3 Calculated, %: C 62,77; H 3,72; N 12,92; F 5,84.
According to the similar method were synthesized the following compounds:
- 5-(2-Fluorobenzoylamino)-1-phenyl-1H-pyrazolo-4-carboxylic acid (4b) with output 71,8% as the white powder with mp.181-183В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 7,15 (1H, t, C6H4F); 7,29 (1H, t, C6H5); 7,54 (7H, m, C6H5 ); 7,81 (1H, s, CH=); 8,14 (1H, m, C6H4F); 11,17 (1H, s, NH); 11,95 (1H, s, COOH). Found, %: C 62,83; H 3,72; N 12,94; F 5,81. C17H12FN3O3 Calculated, %: C 62,77; H 3,72; N 12,92; F 5,84.
- 5-(4-Fluorobenzoylamino)-1-phenyl-1H-pyrazolo-4-carboxylic acid (4c) with output 78,3% as the white powder with mp. 210-213В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 7,37 (2H, t, C6H4F); 7,54 (5H, m, C6H5 ); 7,81 (1H, s, CH=); 8,16 (2H, m, C6H4F); 11,15 (1H, s, NH); 11,77 (1H, s, COOH). Found, %: C 62,79; H 3,74; N 12,88; F 5,85. C17H12FN3O3 Calculated, %: C 62,77; H 3,72; N 12,92; F 5,84.
- 5-(4-Chlorobenzoylamino)-1-phenyl-1H-pyrazolo-4-carboxylic acid (4d) with output 73,3% as the white powder with mp. 223-225В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 7,55 (7H, m, C6H5 ); 7,87 (1H, s, CH=); 7,90 (2H, d, C6H4Cl); 11,23 (1H, s, NH); 11,85 (1H, s, COOH). Found, %: C 59,75; H 3,49; N 12,31; Cl 10,35. C17H12ClN3O3 Calculated, %: C 59,79; H 3,54; N 12,30; Cl 10,37.
- 5-(2-Furoylamino)-1-phenyl-1H-pyrazolo-4-carboxylic acid (4e)
with output 69,2 % as the white powder with mp. 187-190 В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 6,71 (1H, t, C4H3O); 7,34 (1H, d, C4H3O ) 7,54 (5H, s, C6H5); 7,85 (1H, s, CH=); 7,95 (1H, d, C4H3O); 11,10 (1H, s, NH); 11,27 (1H, s, COOH). Found, %: C 60,59; H 3,75; N 14,12. C15H11N3O4 Calculated, %: C 60,61; H 3,73; N 14,14.
6-(3'-Fluorophenyl)-1-phenyl-1H-pyrazolo[3,4-d][1,3]oxazin-4-one (5a)
In a three-neck flask on 100 ml, equipped with a stirrer, thermometer, and a condenser were placed 20,3 g (0,063 mol) 5-(3-fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid Рё 50 ml acetic anhydride. The reaction mixture was stirred under reflux for 2 hours and then cooled to room temperature. The precipitated crystals were filtered, washed with ethyl alcohol and dried under vacuum. Thus was prepared 16,9 g (86,8 %) 6-(3'-fluorophenyl)-1-phenyl-1H-pyrazolo[3,4-d][1,3]oxazin-4-one as a white crystals with mp. 173-176 В°C. Rf 0,85 (toluene-acetone 4:1). NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 7,27 (1H, t, C6H4F); 7,36 (1H, t, C6H4F); 7,54 (5H, m, C6H5 ); 7,79 (1H, m, C6H4F); 8,04 (1H, d, C6H4F); 8,15 (1H, c, CH=). Found, %: C 66,39; H 3,32; N 13,65; F 6,21. C17H10FN3O2 Calculated, %: C 66,45; H 3,28; N 13,67; F 6,18.
According to the similar method were synthesized the following compounds:
- 6-(2'-Fluorophenyl)-1-phenyl-1H-pyrazolo[3,4-d][1,3]oxazin-4-one. (5b)
with output 67,0% as the white powder with mp. 201-204В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 7,27 (1H, t, C6H4F); 7,36 (1H, t, C6H4F ); 7,55 (5H, m, C6H5); 7,79 (1H, m, C6H4F ); 8,04 (2H, d, C6H5); 8,15 (1H, s, CH=). Found, %: C 66,44; H 3,29; N 13,59; F 6,20. C17H10FN3O2 Calculated, %: C 66,45; H 3,28; N 13,67; F 6,18.
-6-(4'-Fluorophenyl)-1-phenyl-1H-pyrazolo[3,4-d][1,3]oxazin-4-one. (5c)
with output 73,9% as the white powder with mp. 220-225В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 7,36 (2H, t, C6H4F); 7,55 (3H, m, C6H5 ); 7,79 (2H, t, C6H4F); 8,04 (2H, d, C6H5); 8,17 (1H, s, CH=). Found, %: C 66,44; H 3,29; N 13,59; F 6,18. C17H10FN3O2 Calculated, %: C 66,45; H 3,28; N 13,67; F 6,18.
-6-(4'-Chlorophenyl)-1-phenyl-1H-pyrazolo[3,4-d][1,3]oxazin-4-one. (5d)
with output 79,4% as the white powder with mp. 231-233В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 7,55 (5H, m, C6H5); 7,75 (2H, d, C6H4Cl ); 8,04 (2H, d, C6H5); 8,10 (1H, s, CH=). Found, %: C 63,11; H 3,14; N 12,99; Cl 10,89. C17H10ClN3O2 Calculated, %: C 63,07; H 3,11; N 12,98; Cl 10,95.
-6-(2'-Furoyl)-1-phenyl-1H-pyrazolo[3,4-d][1,3]oxazin-4-one. (5e)
with output 73,9 % as the white powder with mp. 211-213 В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 6,74 (1H, t, C4H3O); 7,14 (1H, d, C4H3O); 7,55 (3H, m, C6H5); 7,97 (1H, d, C4H3O); 8,07 (2H, d, C6H5); 8,16 (1H, s, CH=). Found, %: C 64,49; H 3,32; N 15,17. C15H10N3O3 Calculated, %: C 64,52; H 3,25; N 15,05.
5-(3'-Fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid (4"-fluorophenyl)amide (6a). In a two-neck flask on 50 ml, equipped with a stirrer, thermometer, and a condenser were placed 1,0 g (3 mmol) 6-(3'-fluorophenyl)-1-phenyl-1H-pyrazolo[3,4-d][1,3]oxazin-4-one и 0,40 g (0,34 ml, 3,6 mmol) freshly distilled 4-fluoroaniline. The reaction mixture was heated at the temperature 100 – 120 °C during 1 hour. Then the reaction mixture was cooled, 10 ml of ethyl alcohol was added and the mixture was heated under reflux for 5 minutes. The resulting homogenous solution was cooled on an ice bath and the precipitated crystals were filtered, washed with ethyl alcohol and dried. Thus was prepared 1,15 g (91,3 %) 5-(3'-fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid (4"-fluorophenyl)amide as a grey crystals with mp. 210-215 °C. Rf 0,25 (toluene-acetone 4:1). NMR spectrum 1H (20% in CDCl3), δ, m.d.: 7,17 (1H, t, C6H5); 7,45 (3H, m, C6H5); 7,58 (3H, m, C6H5 ); 7,68 (3H, m, C6H5); 7,70 (1H, d, C6H5); 8,42 (1H, s, -CH=); 10,06 (1H, s, NH); 10,58 (1H, s, NH). Found, %: C 64,49; H 3,32; N 15,17; F 9,05. C23H16F2N4O2 Calculated, %: C 64,52; H 3,25; N 15,05; F 9,08.
According to the similar method were synthesized the following compounds:
-5-(2'-Fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid (1"-naphthyl)amide (6b) with output 87,2 % as the grey powder with mp. 215-220 В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 7,30 (2H,m,C6H5), 7,48 (1H, m, C6H5), 7,55 (6H, m, C6H5), 7,65 (4H, m, C6H5), 7,87 (1H, d, C6H5), 7,98 (1H, d, C6H5), 8,03 (1H, d, C6H5), 8,55 (1H, s, -CH=), 10,10 (1H,s, NH), 10,50 (1H, s, NH). Found, %: C 71,97; H 4,29; N 12,39. C27H19N4O2 Calculated, %: C 71,99; H 4,25; N 12,44.
-5-(4'-Chlorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid (4"-fluorophenyl)amide (6c) with output 91,1% as the light-yellow powder with mp. 210-213 В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 7,17 (1H,t, C6H5), 7,45 (3H, m, C6H5), 7,58 (3H, m, C6H5), 7,68 (3H, m, C6H5), 7,70 (1H, d, C6H5), 8,42 (1H, s, -CH=), 10,06 (1H,s, NH), 10,60 (1H, s, NH). Found, %: C 63,52; H 3,69; N 12,91; F 4,35; Cl 8,17. C23H16FClN4O2 Calculated, %: C 63,53; H 3,71; N 12,88; F 4,37; Cl 8,15.
-5-(4'-Fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid (2"-ethoxycarbonylphenyl)amide (6d) ) with output 73,9% as the white powder with mp. 180-183 В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 1,20 (3H, t, CH3), 4,08 (2H, q, CH2), 7,20 (1H, t, C6H5), 7,36 (3H, m, C6H5), 7,50 (2H, t, C6H5), 7,58 (3H, m, C6H5), 7,96 (3H, m, C6H5), 8,28 (1H, s, -CH=), 8,55 (1H, d, C6H5), 10,70 (1H, s, NH), 11,29 (1H, s, NH). Found, %: C 66,13; H 4,45; N 11,89; F 4,01. C26H21FN4O4 Calculated, %: C 66,10; H 4,48; N 11,86; F 4,02.
-5-(3'-Fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid -(4"-chlorophenyl)amide (6e) with output 92,1% as the grey powder with mp. 208-210В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 7,50 (9H, m, C6H5), 7,57 (4H, m, C6H5), 8,43 (1H, s, -CH=), 10,15 (1H, s, NH), 10,65 (1H, s, NH). Found, %: C 63,49; H 3,71; N 12,92; F 4,36; Cl 8,13. C23H16FClN4O2 Calculated, %: C 63,53; H 3,71; N 12,88; F 4,37; Cl 8,15.
-5-(2'-Fluorobenzoylamino)-1-phenyl-1H-pyrazol-4-carboxylic acid -(2", 4"-dimethoxyphenyl)amide (6f) with output 77,5% as the dark-grey powder with mp. 180-183В°C. NMR spectrum 1H (20% in CDCl3), Оґ, m.d.: 3,55 (3H, s, OCH3), 3,85 (3H, s, OCH3), 6,52 (1H, d, C6H5), 6,60 (1H, s, C6H5), 7,35 (1H, m, C6H5), 7,45 (1H, m, C6H5), 7,60 (6H, m, C6H5), 7,78 (1H, d, C6H5), 8,33 (1H, s, -CH=), 8,95 (1H, s, NH), 10,60 (1H, s, NH). Found, %: C 65,23; H 4,61; N 12,15; F 4,10. C25H21FN4O4 Calculated, %: C 65,21; H 4,60; N 12,17; F 4,13.
References
- S. V. Nielsen, E. B. Pedersen, Chem. Scr, 23, 1984, p. 240-244.
- M. M. Ghorab, Acta Pharmaceutica (Zagreb), 50, 2000, p. 93-110.
- E. R. Bendary, F. A. Badria, Arch. Pharm., 333, p. 99-103.
- C. Jyh-Haur, S. Kak-Shan, H. Tsu-An, T. Chia-Liang, et al., Bioorg. Med. Chem. Lett., 14, 2004, p. 2519-2531.
- P. P. Gupta, R. C. Srimal, K. Avasthi, T. Chandra, D. S. Bhakuni, Indian J. Exp. Biol., 33, 1995, p. 38-40.
- S. A. El-Feky, Z. K. Abd el-Samii, Pharmazie, 51, 1996, p. 540-543.
- G. Szilagyi, E. Kasztreiner, L. Tardos, E. Kosa, L. Jaszlits, G. Cseh, et al., Ger. Offen. 2,825, 906 (Cl. CO 7D 403/04), (1979), Hung. Appl. 1, 373, 1977; Chem. Abstr., 90, 152224x (1979).
- B. Dumaitre, N. Dodic, J. Med. Chem., 39,1996, р. 1635 – 1644.
- M. M. Ghorab, F. A. Ragab, E. Noaman, H. I. Heiba, S. A. Aboulmagd, Arzneimittelforschung, 59 (2), 2009, р. 96–103.
- I. Devesa, M. J. Alcaraz, A. Riguera, M. L. Ferrandis, Eur. J. Pharm., 488, 2004, p. 225-230.
- J. W. Marsico, J. P. Joseph, US: 3864,359 (Cl. 260–310R; CO 7d), 4 February 1975, Appl. 248, 990, 1 May 1972; 6 pp; Chem. Abstr., 82, 170936v (1975).
- M. Chauhan, R. Kumar, Bioor.& Med.Chem., 21, 2013, p. 5657-5668.
- K. Yang, X. He, H. Choi, Z. Wang, D. H. Woodmansee, H. Liu, Tetr.Lett., 49, 2008, p.1725-1728.
Recommended for publication by Prof. A.F. Eleev
Fluorine Notes, 2014, 95, 3-4
