Received: September, 2013
Fluorine Notes, 2014, 93, 1-2
The reactions of alkylation, arylation, alkenylation, and alkynylation of polyfluoroarenes and -hetarenes by their aromatic rings
T.D.Petrova, V.E.Platonov
N.N.Vorozhtsov Novosibirsk Institute of Organic Chemistry, Siberian Branch, Russian Academy of Sciences, pr. Akademika Lavrent,eva 9, Novosibirsk, 630090 Russia
e-mail: petrova@nioch.nsc.ru, platonov@nioch.nsc.ru
Abstract: Review considers the reactions of alkylation, arylation, alkenylation, and alkynylation of polyfluoroarenes and –hetarenes occurring in the aromatic ring and leading to the formation of CAr-C bond. Review contains information on the traditional methods of conducting such transformations which include, for example, reactions of polyfluoroarenes with electrophilic, nucleophilic, radical reagents. Along with these methods, reactions using of metal complex catalysis are considered. The application of metal complex catalysis expands the area the substrates used. This method includes cross-coupling reactions between metal or metal compounds and aryl halides catalyzed by transition metal complexes with various organic ligands. Kumada, Negishi, Suzuki, Stille, Hiyama, Heck, and Sonogashira reactions are presented. For polyfluoroarenes and –hetarenes CAr-Hal bond transformations involves also CAr-F bond.
Keywords: polyfluoroarenes and –hetarenes, alkylation, arylation, alkenylation, alkynylation, metal complex catalysis, palladium catalysts, cross-coupling reactions.
III. Transformation involving CAr – Hal bond (Br, Cl, I)
1. Reactions with Na- and Mg- organic derivatives
2. Reactions with B- organic compounds (Suzuki reaction)
3. Reactions c Cu-organic compounds and copper catalysis
4. Production and transformations of Zn-organic derivatives
5. Reaction with Sn-organic derivatives (Stille reaction)
6. Reaction of Si-organic derivatives (Hiyama reaction)
7. Reactions of Li-organic compounds
8. Alkenylation reactions
9. Alkynylation reactions of polyfluorohalogenated arenes (Sonogashira reaction)
In this chapter we consider alkylation, arylation (hetarylation), alkenylation and alkynylation of polyfluorohalogenated arenes (Hal = Br, Cl, I), that selectively occur by CAr-Hal bond with substitution of the halogen with an appropriate group. Most of those reactions are based on the direct nucleophilic substitution of halogen in the presence of various catalysts, sometimes via the formation of intermediate polyfluorinated organometallic compounds which undergo further transformations to result in final products.
1. Reactions with Na- and Mg- organic derivatives
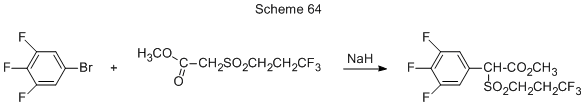
Methyl-(3’,4’,5’-trifluorophenyl-3,3,3-trifluoropropylsulfonyl)acetate is produced from 3,4,5-trifluorobromobenzene and methyl-(3,3,3-trifluoropropylsulfonyl)acetate in the presence of sodium hydride (Scheme 64) [158a].
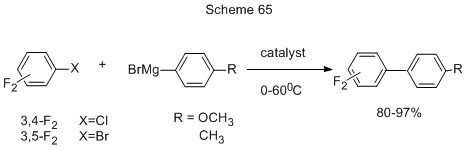
A convenient method for the production of asymmetric polyfluorinated biphenyls is the coupling of phenylmagnesium bromides with polyfluorochloro- or - bromobenzenes using for effective catalysts some combinations of N-heterocyclic carbene (NHC) with fluorides of Fe, Co, Ni (Scheme 65) [159].
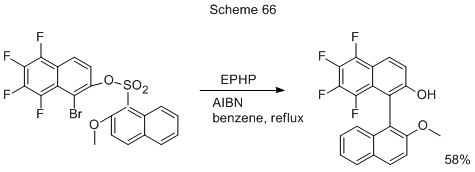
The attempts to produce polyfluorinated methoxyderivatives of 1,1'-binaphthyls via the coupling of 1-bromo-2-methoxy-5,6,7,8-tetrafluoronaphtalene with 2-methoxy-1-naphtylmagnesium bromide or with 1-Li-naphthyl derivative in the presence of NiCl2(PPh3)2, or starting with the corresponding 1-iodonaphtalene derivative in the presence of Pd(PPh3)4, were not very successful because of low yields (<10%) of the target binaphthyl [40]. However, the problem was successfully solved by intramolecular radical cross-coupling in polyhalogenated naphtyl ester of 2-methoxy-naphthalene-1-sulfonic acid (Scheme 66) under the action of 1-ethylpiperidine hypophosphite (EPHP), and α,α’-azobis-isobutyrodinitrile (AIBN) [40]. It is assumed that a radical-induced 1,5-ipso-substitution occurs via a spirocyclic intermediate capable of rearomatization by the loss of SO2.
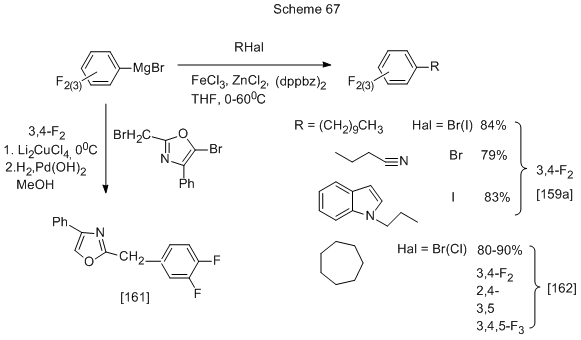
Another variant of the reaction with Mg-organic derivatives involves the formation of Mg-organic compound with halogen taken from the initial polyfluorophenylhalogenarene that further reacts with its precursor, or with another halogenated derivative. For example, in the treatment of 3,4- and 2,4-difluorobromobenzene with magnesium in THF, and subsequent addition of CoCl2 in catalytic amount (5 mol%) the coupling reaction occurs, and corresponding tetrafluorobiphenyl is formed with yield 48% or 65% [160]. Some other examples are shown in Scheme 67.
Difluorophenylmagnesium bromides obtained from isomeric difluorobromobenzenes (3,4-F2 [163], 3,5-F2 [122, 164, 165], 2,5-F2 [166]), 2,5-difluorophenylmagnesium chloride [167], polyfluoroarylmagnesium bromides obtained from 2,4,5-trifluorobromobenzene [168], 2,3,5,6-tetrafluoro-4-bromopyridine, and bromopentafluorobenzene [168, 169] react with various carbonyl compounds involving their carbonyl groups and result in corresponding oxyalkylation products.
2. Reactions with B- organic compounds (Suzuki reaction)
Suzuki reaction has already been discussed in Chapter II 2.3. Here, this reaction is shown with reference to cases, when halogen (Br, Cl, I) of original polyfluorohalogenarene participates in the reaction. Some examples with aryl- and hetarylboronic acids are shown below (Scheme 68).


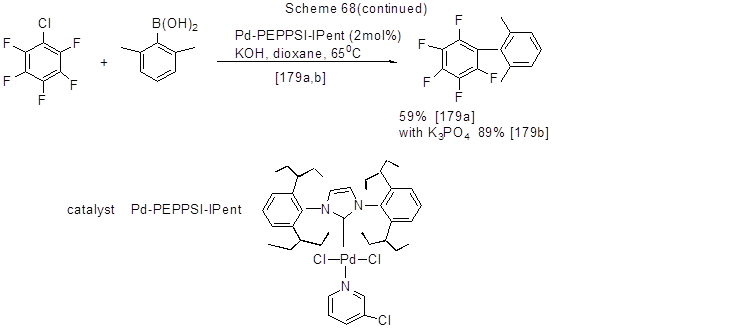
The examples of reactions with fluorinated arylboronic acids include the reaction of 3-fluorophenyl- and 4-fluorophenylboronic acids with 2,4- difluoroiodobenzene in the presence of Na2CO3 and Pd(PPh3)4, which results in the formation of 2,3',4-trifluoro- and 2,4,4'- trifluorobiphenyls with yields of 94 % and 88 % correspondingly [ 73].
3. Reactions c Cu-organic compounds and copper catalysis
Cross-coupling of various aryl groups is the most important step in the manufacture of perfluorinated branched oligophenylenes, new electron-transport materials to be used in diodes, and in this respect the most satisfactory results are due to Cu-organic chemistry [180]. Scheme 69 shows a route to one type of dendrimers that involves Cu-organic derivatives.
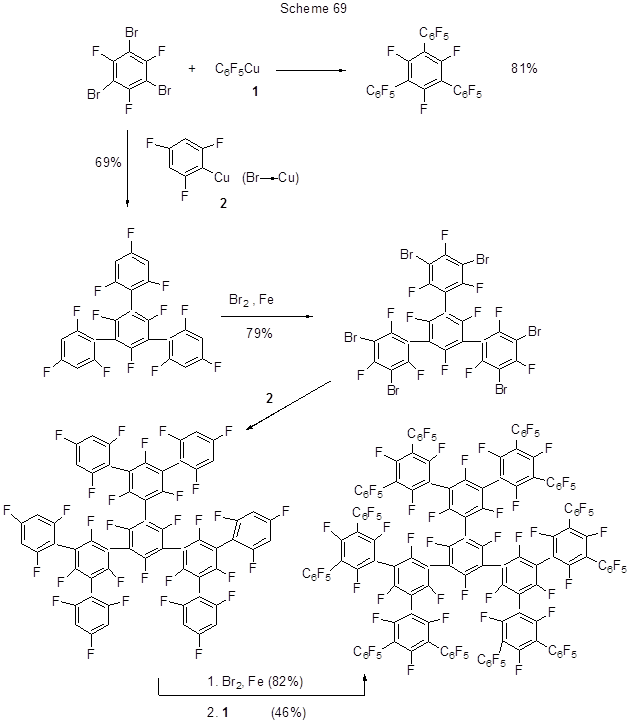
5,5',6,6',7,7',8,8'-Octafluoro-2,2'-dimethoxy-1,1'-binaphthyl (1) (Scheme 70) was produced from 1-bromo-2-methoxy-5,6,7,8-tetrafluoronaphtalene (2) through the cross-coupling in the presence of copper, by the Ullman reaction with yield 85% [181]. The attempt to use this path for the production of partially fluorinated binaphthyls, in particular 5,6,7,8-tetrafluoro-2,2'-dimethoxy-1,1'-binaphthyl (3) by the cross-coupling of original bromotetrafluorometoxynaphtalene 2 with 1-bromo-2-methoxy-naphthalene in the presence of copper resulted in the formation of above octafluorodimetoxybinaphtyl 1 with yield of 85%, while the target tetrafluorodimetoxybinaphtyl 3 was produced with 5% yield only (Scheme 70) [40].
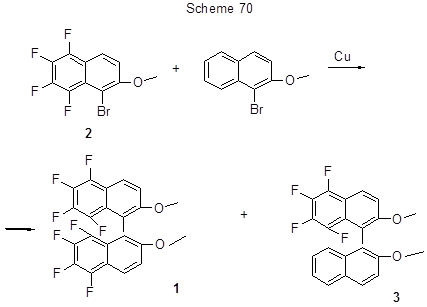
The Ullmann reaction of 1-bromo-2-methoxy-5,6,7,8-tetrafluoronaphtalene 2 with 2-substituted 1-bromonaphthalenes containing acceptor substituents (COOR, CHO) at the position 2, proceeds more successfully. The yield of tetrafluorobinaphtyls is 27-48%. To obtain higher yields of 2,2’-substituted 5,6,7,8-tetrafluoro-1,1'-binaphthyls some oxidative coupling methods were applied, which unlike the Ullmann reaction are more successful with electron-donating substrates. Thus, in the presence of 10 mol% Cu (OH) Cl. TMEDA catalyst, the coupling of 4,5,6,7-tetrafluoro-2-naphthol with 2-naphthol resulted in the target tetrafluorobinaphtyl-2,2'-diol with yield 40%. In the presence of CuI, KI, and palladium catalyst the reaction between difluorobromophenyl derivative of dimethylpirazole and methylimidazole-1 involves the substitution of bromine by a heterocyclic fragment (Scheme 71) [182].
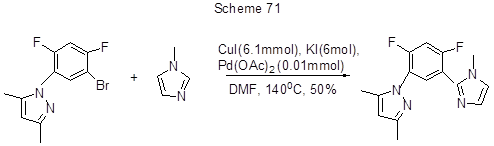
The alkenylation of bromopentafluorobenzene is conducted in the presence of cadmium and CuCl (Scheme 72) [183].
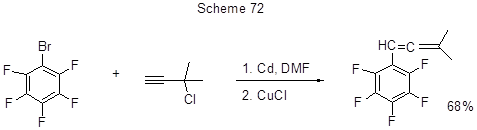
4. Production and transformations of Zn-organic derivatives
Allylpolyfluoroarenes and –hetarenes result from the interaction of Zn-organic reagents, produced from polyfluorobromo- or - chloroarenes and hetarenes under the action of zinc, with allylbromide or allylchloride [184]. The general transformation mechanism is shown in Scheme 73.
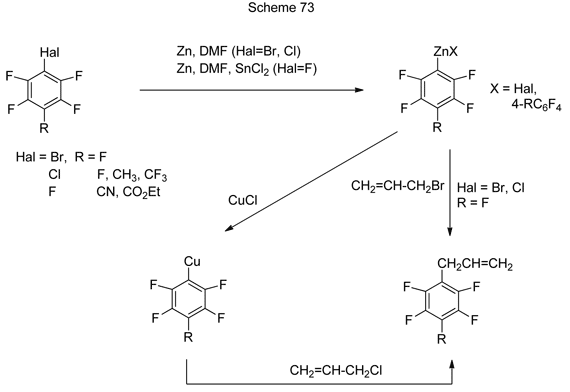
The reaction between polyfluorinated Zn-organic reagents, produced from C6F5X (X = Br, Cl), and allylbromide involves nucleophilic substitution of bromine atom in allylbromide with C6F5-group. Allyl derivatives are also formed with good yields in the reaction between polyfluoroaryl zincorganic reagents, produced from chloropolyfluoroarenes, with allylchloride in the presence of CuCl. The catalysis with copper salts is apparently due to the formation of Cu-organic compounds from relevant Zn-organic reagents, which further interact with allylchloride. The conversion of 4-chlorotetrafluoropyridine and 5-chlorononafluoroindane under the action of Zn/DMF and that of allylchloride in the presence CuCl [184] occur in the similar manner.
The procedure for the preparation of high purity decafluorobiphenyl with yield 70-74% by heating bromopentafluorobenzene with Zn and Cu (II) acetate is also disclosed [169, C.117-118].
5. Reaction with Sn-organic derivatives (Stille reaction)
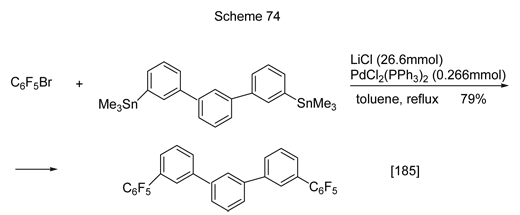
The arylation or hetarylation of brompolyfluorobenzenes their cross-coupling with tin-arenes or tin-hetarenes in the presence of palladium catalysts (Stille reaction) is used. One example is shown in Scheme 74 [185].
Stille reaction is used for a method of synthesis polyfluorinated polythiophene derivatives, those being promising semiconductor materials (Scheme 75) [186].
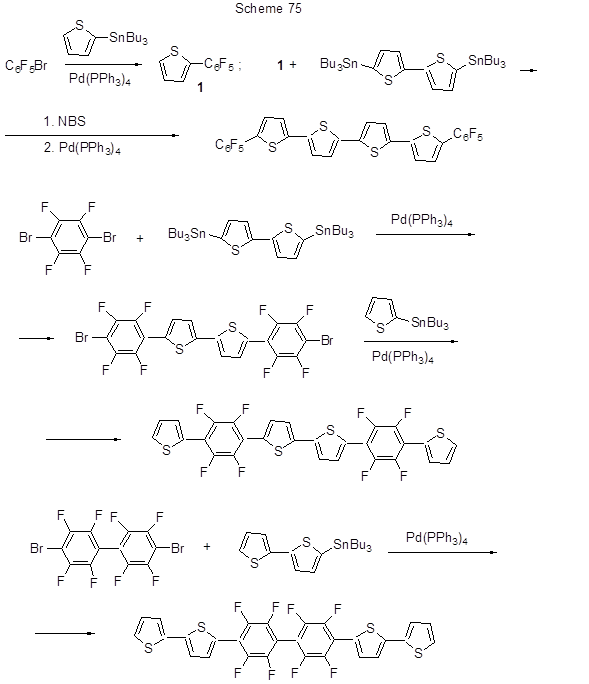
The reaction of thiophene Sn-derivatives with polyfluorinated iodarenes proceeds similarly (Scheme 76) [187].
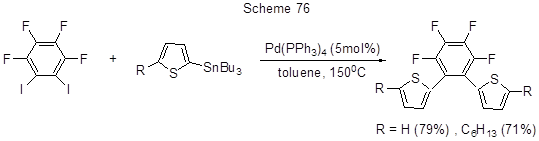
Another example of polyfluoroarylation by Stille reaction is shown in Scheme 77 as an intermediate step in the synthesis of N-substituted 5-arylimidazol-2-amines [188].
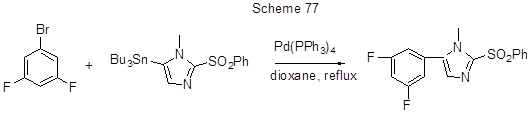
Stille reaction does not occur between 1-bromo-2-methoxy-5,6,7,8-tetrafluoronaphtalene and (2-methoxynaphthyl-1)tributyl tin [40]. The reactions between aromatic bromodifluoroderivatives and tributyl-(pyridine-2-yl) tin are presented in Scheme 78.

Aromatic derivatives containing electrophilic sulfonyl groups and fluorines (left reaction in Scheme 78 [189]) are of interest as ligands for new iridium complex with various applications. The palladium catalyst Pd(AsPh3)4, used in Stille reaction, is produced in situ from [Pd2(dba)3] and AsPh3. Other examples of arylation according to Stille reaction, including reactions of polyfluorinated Sn-organic derivative and non-fluorinated halogenarene, are shown in Scheme 79.
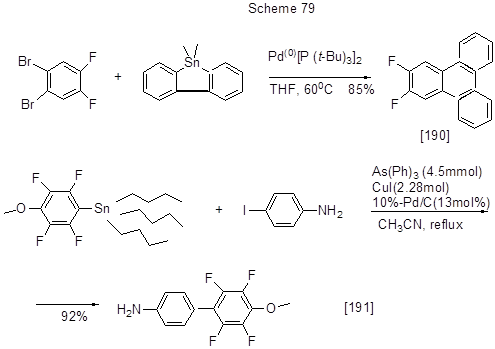
Vinyl group is introduced at position 6 of 5,7-difluoro-6-bromoquinoline under the action of tributylvinyl tin using Pd(PPh3)4 and microwave irradiation [192], while it is introduced at position 5 of 2,3,4-trifluoro-5-iodo-benzoic acid by the reaction of the reactant with acid with the help of Pd2(dba)3 and 2-furylphosphine used for catalysts (the yield of vinyl derivative is 83%) [193].
The possibility of using organic tin reagent in other reactions, e.g., in intramolecular radical arylation, is demonstrated in the case of conversion of difluorosubstituted C-allyl adduct of a Schiff base produced from isatin, to ortho-, ortho-disubstituted difluorobiphenyl with internal 8-membered lactam ring, interesting due to its physiological activity, the said conversion occurs under the influence of tributyltin hydride in the presence of AlBN (Scheme 80) [194].
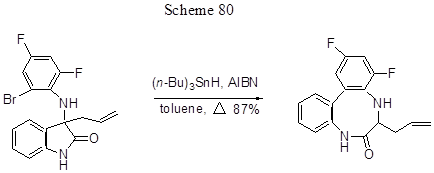
6. Reaction of Si-organic derivatives (Hiyama reaction)
Cross-coupling of iodopentafluorobenzene with 2-(trimethylsilyl)-furan in the presence of Pd-catalyst and a base by a type of Hiyama reaction results with 54% yield in 2-pentafluorophenylfuran (Scheme 81) [195].
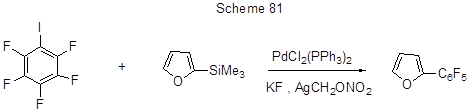
The paper [196, Scheme 82] illustrates the possibility of arylation of ortho- bromophenols, including those polyfluorinated, protected by silyl groups, due to the fact that this group is an effective phenyl group donor. It is assumed that in the course of Pd-catalyzed intramolecular arylation an oxasilocyclic intermediate is formed, and its silyl group is further eliminated resulting in a biphenyl derivative.
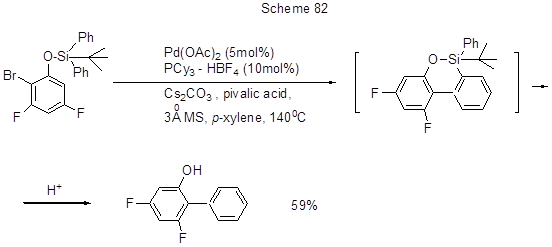
7. Reactions of Li-organic compounds
The reaction of 1,2-dibromo-3,4,5,6-tetrafluorobenzene with butyllithium and TiCl4 results in arylation to 2,2'-dibromooctafluorobiphenyl [197], while that of 4-bromo-2,6-difluoroiodobenzene with butyllithium and further with CuF2 results in 4,4'-dibromo-2,2',6,6'-tetrafluoro-1,1'-biphenyl with yield 79% [67].
The introduction of oxyalkyl groups instead of bromine or iodine into polyfluoroarylbromides or –iodides is conducted by the reaction of corresponding Li-organic compounds derived from those halides, with carbonyl derivatives or oxides. This transformation occurs when Li-organic derivatives of bromopentafluorobenzene or bromononafluorobiphenyl react with octafluorofluorenone [198], those of 1-iodo-3,5-difluorobenzene react with acetaldehyde in the presence of tetramethylpiperidine (yield 47%) [80], those of 2,3,5 trifluorobromobenzene, or 3,4-difluoro-2-methylbromobenzene, or 2-methyl-3,5-difluoroiodobenzene, or 2,3,5,6-tetrafluorobromobenzene react with propylene oxide in the presence of boron trifluoride etherate (hydroxyalkyl derivative yields are 83%, 71% , 32%, and 62%, correspondingly) [199], and those of 3,4,5-trifluorobromobenzene react with cyclohexene oxide in the presence of boron trifluoride etherate as well [200].
In some cases, Li-organic compounds derived from polyfluorohalogenated arenes were applied in transmetalation reactions to prepare other polyfluorinated elementarenes, e.g., organoboron derivatives which further entered various transformation processes. Reactions of those boron derivatives are discussed in Section 2.
8. Alkenylation reactions
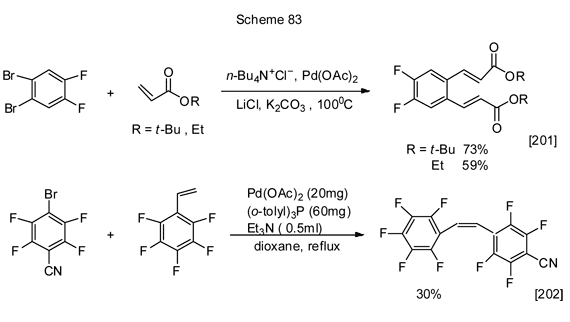
The intermolecular reactions of polyfluoroarenes’ alkene derivatives formation from brompolyfluoroarenes following the Mizoroki-Heck reaction are exemplified below (Scheme 83).
The reaction may also follow an intramolecular mechanism as well (Scheme 84).
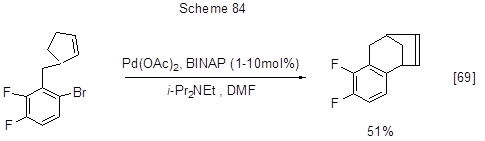
Intramolecular reductive version of Mizoroki - Heck reaction is also possible, this being essentially hydroarylation of alkene at α- or β- position.
Therefore, dihydroindenoisoquinolines, including those difluorinated, interesting in terms of their biological activity, are proposed to obtain from a 3- component system ( imine, benzoyl chloride, olefin Sn-derivative) in a two-step process, the first of which is Cu (I)-catalyzed benzoylation of imine to amide, and coupling with olefin, and the second step is Pd(0)-catalyzed α-arylation that is intramolecular Mizoroki - Heck reaction with the formation of an assumed intermediate, in which the intramolecular β-arylation is proceed to form dihydroindenoisoquinoline (Scheme 85) [ 203].
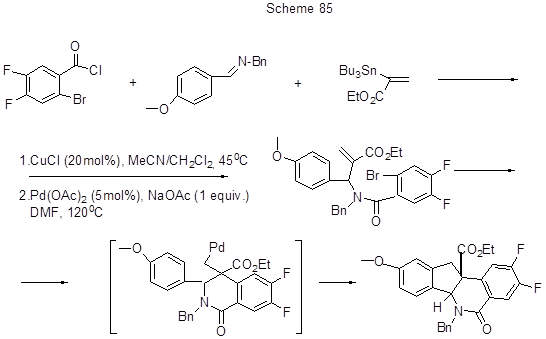
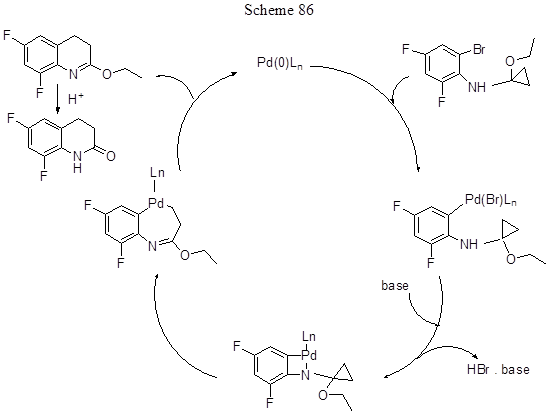
Another intramolecular reaction (not Mizoroki - Heck reaction, but that with bromine atom), allowing ultimately to obtain difluoroderivative of 3,4-dihydro-2(1H)quinolinone with 70% yield, involves expanding of the cyclopropane ring of N-(1'-ethoxy)cyclopropyl-2-bromo-4,6-difluoroaniline (Scheme 86) [204]. The reaction mechanism apparently involves arylbromide oxidative addition to Pd(0) to form a 4-membered azapalladium cycle, this cycle rearrangement with the cyclopropane ring opening and conversion to 7-membered ring, and subsequent reductive elimination with Pd(0) regeneration and isolation of 2-ethoxy-6,8-difluoro-3,4-dihydroquinoline. The used catalyst system consists of Pd2(dba)3 (1.5mol%), dicyclohexyl-(2',4',6'-trispropilbiphenyl-2-yl)phosphine (7.5mol%) in DMF, and K2CO3 base (1.5equiv.), the reaction temperature is 95oC.
9. Alkynylation reactions of polyfluorohalogenated arenes (Sonogashira reaction)
Sonogashira reaction in its classic version, i.e. cross-coupling of a halogenated derivative with alkyne in the presence of Pd-catalyst, copper salt and addition of base, was applied to produce hybrid oligomers with a polyfluorinated central fragment, those being of interest in the study of optical and electrochemical properties of π-conjugated materials (Scheme 87 [205]), and as one of the steps in the synthesis of cholesterol absorption inhibitors of the series of 3-(arylpropynyl)substituted-2-azetidinones (Scheme 88 [206]).

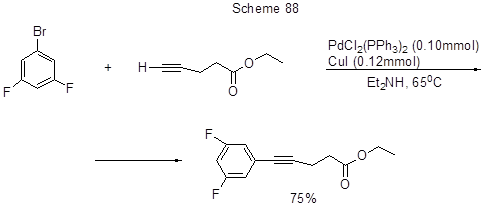
Copper catalysis was successfully replaced with microwave irradiation (Scheme 89) [207].
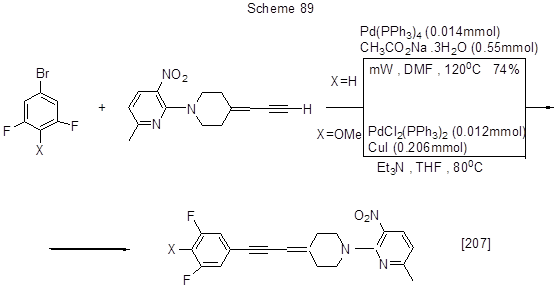
Some other examples of Sonogashira reaction are shown in Scheme 90.

For iodopentafluorobenzene cross-coupling with phenylacetylene in the presence of various catalyst systems resulting in the formation of 1-phenyl-2-pentafluorophenylacetylene see [211]. Alkynylation of 3,5-difluoropolybromopyridine derivatives according to Sonogashira reaction is shown in Scheme 91 [212].
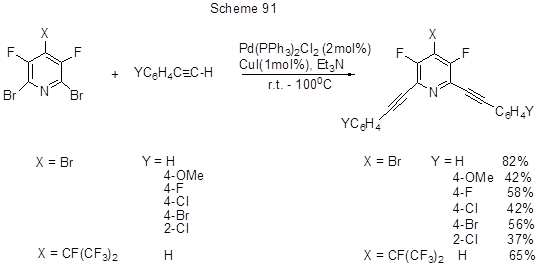
It is assumed that Pd-catalyst is introduced at a weaker CAr-Br bond, but the reaction involves bromine atoms at positions 2, 6, i.e. at ortho-positions to the nitrogen atom, in contrast to nucleophilic substitution that occurs at in position 4. The authors suggest that the nitrogen atom charge in the transition state may provide some effect on the position of substitution.
IV. Transformation involving CAr–F bond
1. Reactions of nucleophilic substitution of fluorine with C-nucleophiles
1.1 Reactions with Na-organic substances
1.2 Reactions with Li-organic derivatives
1.3 Reactions with Mg-organic derivatives
1.4 Effect of Si-organic derivatives in the presence of catalysts generating fluoride-ion.
2. Transformations of CAr–F bond involving its activation
The processes for alkylation, arylation (hetarylation) or introduction of groups with multiple C-C bonds in polyfluoroarene molecule are realized with the participation of CAr-F bond. They make use of various methods, especially nucleophilic substitution of fluorine bound to a carbon of aromatic ring, which is the most usual technique with halogenated derivatives, including polyfluorinated arenes. The best studied in this respect are reactions with C-nucleophiles generated from various organometallic compounds (Li, Na, Mg, Zn, Sn). However, in the last decade the chemists that study organic halogen derivatives, including halogenated arenes, developed novel approaches to involvement of halogens into transformation by the various methods for activation of CAr-Hal bond. This was in order to increase both the reaction yield and the regioselectivity of those transformations. Fluoroarenes in this regard were no exception. A recently published review by the Japanese authors " C-F Activation in Organic Synthesis" [213] summarizes the results obtained in this direction and provides a useful list of publications related to this issue.
1. Reactions of nucleophilic substitution of fluorine with C-nucleophiles
1.1. Reactions with Na-organic substances
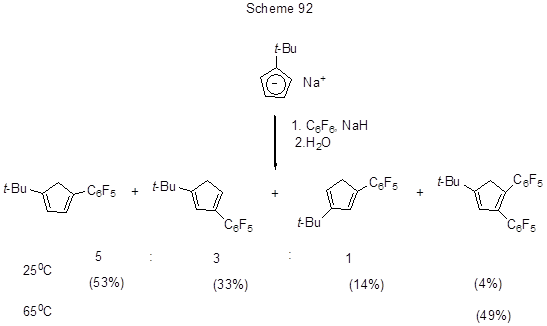
Na-organic compounds were applied for nucleophilic substitution of fluorine atoms in polyfluoroarenes as the source of C-anions. Thus, the interaction of hexafluorobenzene with a Na-derivative of t-butylcyclopentadiene resulted in a mixture of isomeric pentafluorophenyl-t-butylcyclopentadienes and 1,2-bis(pentafluorophenyl)-4-t-butyl-cyclopentadiene (Scheme 92) [214].
In a similar reaction of octafluorotoluene C-nucleophile attacks fluorine atom at the para-position to the CF3-group [215]. 3,4-Difluoronitrobenzene reacts with sodium malonate to result in 2-fluoro-4-nitrophenylmalonate which, under the action of MgCl2, easily undergoes double decarboxylation to result in 2-fluoro-4-nitrotoluene [216]. At those conditions 2,4-difluoronitrobenzene is converted to 3-fluoro-6-nitrotoluene (73%). This reaction opens the rout to the production of methylated nitroaromatic compounds.
1.2 Reactions with Li-organic derivatives
Li-Organic compounds are traditional C-nucleophilic reagents. For example, 1-hydroxy-2-methylpropyl group is introduced onto 2-position of 4-t-butoxy-2,3,5,6-tetrafluoropyridine through the reaction of this compound with Li-CH2CHCH3-CH2OSi(CH3)2t-Bu. The product yield is 63% [217]. Under the action of n-butyl- or phenyl- lithium derivatives on 1,4-bis(dimesitylphosphino)-2,3,5,6-tetrafluorobenzene its fluorines at 2, 5 positions are replaced with n-butyl- or phenyl- groups respectively [218]. Intramolecular transformation occurs under the action of n-BuLi on 2-bromo-2’-pentafluorophenylbiphenyl or 2-bromo-2’-pentafluorophenyl-1,1’-binaphtyl [219]. In so doing 1,2,3,4-tetrafluorotriphenylene or 7,8,9,10-dibenzo-1,2,3,4-tetrafluorotriphenylene are formed correspondingly with high yields. Depending on the amount of Li-derivative the final phenylenes can be prepared sequentially or in a one-pot scheme (Scheme 93).
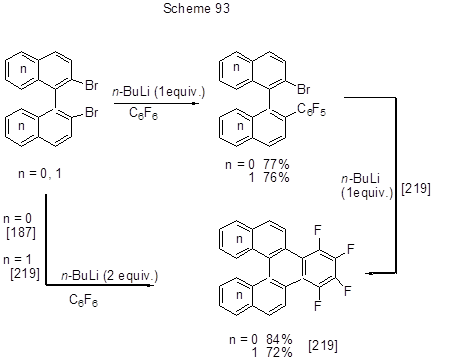
The nucleophilic substitution of 2-pentafluorophenyl-1-phenyltetrafluoroethanes with Li-organic compounds occurs only at the para-position to its tetrafluoroethyl group (Scheme 94) [220].
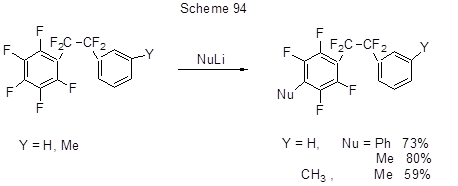
The reactions with Li-organic derivatives were used in the synthesis of compounds with promising semiconductor properties, which contain polyfluorinated aromatic rings in their molecular structures. This is true for the production of polycyclic substances, particularly, terphenyls. Li-derivatives were then synthesized by H→Li or Br→Li exchange and SNAr nucleophilic substitution of fluorine in polyfluoroarene was then conducted (Scheme 95). The resulting polyfluorinated terphenyls cyclised to form polyfluorinated benzo-bis(benzothiophenes), in which fluorines underwent nucleophilic replacing with ethinyl groups under the action of Li-derivatives of ethylacetylene (Scheme 95) [221].
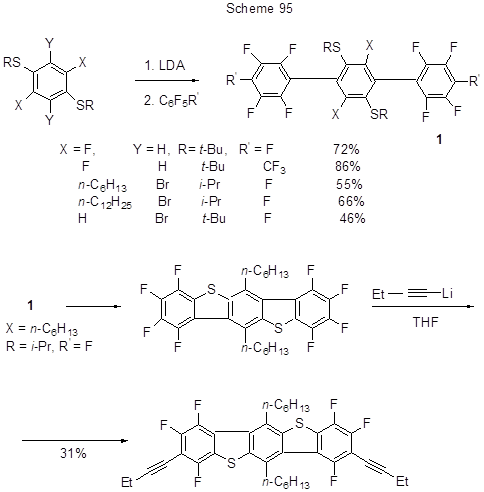
For some examples of vinylation during the nucleophilic substitution of fluorine atom under the action of Li-ethylene see [40] (Scheme 96).
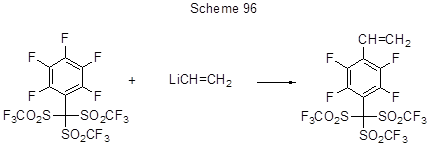
The preparation of ligands with polyphenylene structures is conducted through replacing para-fluorines in terphenyl C6F5-moieties under the action of Li-organic derivatives, and those fragments were introduced into terphenyls reacting hexafluorobenzene with Li-terphenyl derivatives (Scheme 97) [185].
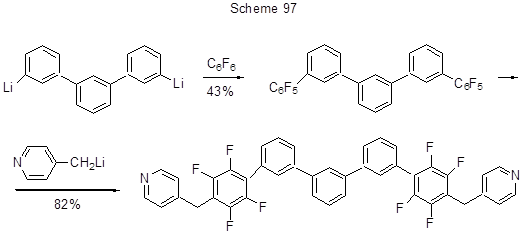
In terms of semiconductor materials, polycycles with various elements embedded in their structures are of major interest. Scheme 98 shows an example of a 9-tinanthracene derivative production with the help of Li-organic compound [222].
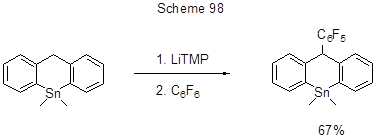
A convenient, high-yield pathway to the synthesis of promising oligophenylenes with perfluorinated moieties involves, along with other transformations, at its first step nucleophilic substitution of aromatic fluorine in decafluorobiphenyle followed then by nucleophilic substitution of para-fluorines in thus generated polyphenylenes under the action of Li-derivatives of various mono-, di- or poly- aryls [223]. Scheme 99 exemplifies such transformation with the use of di-Li-arene.
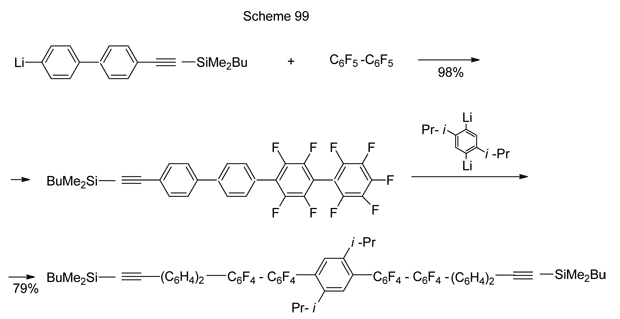
Among the fluorine nucleophilic substitutions in polyfluoroarenes under the action of alkyl- or aryl- lithium compounds one may specify the synthesis 4'-bromo-2,3,4,5,6-pentafluorobiphenyl from hexafluorobenzene and 4-bromophenyllithium [224], (4-t-butylthiophenyl)-nonafluorobiphenyl from decafluorobiphenyl, and 4-t-butylthiophenyl lithium [225]. para-Aryl- and para-alkyl-tetrafluorophenyl-bis(trifluoromethylsulfonyl)methanes are produced through the substitution of para-fluorines in pentafluorophenyl-bis(trifluoromethylsulfonyl)methane under the action of aryl- or alkyl- lithium derivatives. The reaction product in which R contains a phenyl radical with polystyrene moiety at position 4 involves highly acidic C-H bond, and presents a novel Brønsted carbonic super-acid that proved to be an efficient catalyst in the alcohol acylation by acid anhydrides (Scheme 100) [226].
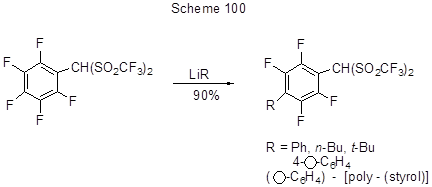
In the studies devoted to the production of semiconductor materials the reactions between polyfluoroarenes and heterocyclic Li-derivatives were used. For instance, the reaction of 2-thienyllithium or 4-hexyl-2-thienyllithium with hexafluorobenzene is the first step in the obtaining of oligothiophenes with a central tetrafluorophenylene link (Scheme 101) [227].
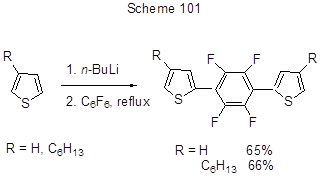
Benzodithiophenes or -diselenophenes functionalized with terminal polyfluoroaryl groups were produced by SNAr reaction of their bis-lithium derivatives with polyfluoroarene (Scheme 102) [228].

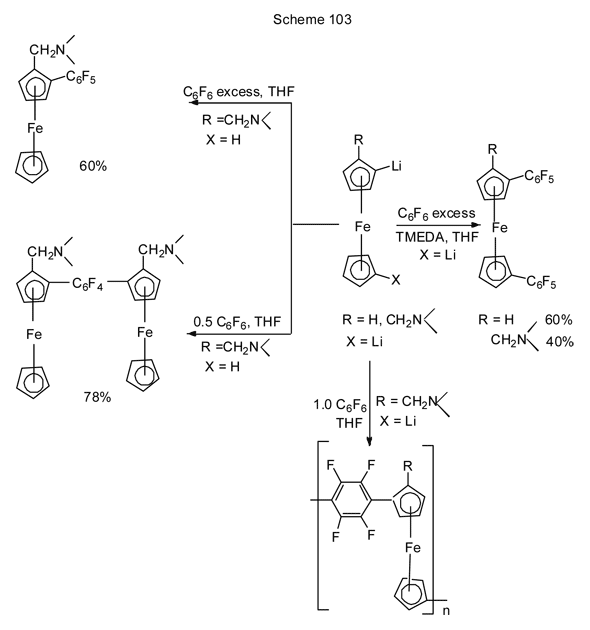
Nucleophilic substitution of fluorine in polyfluoroarenes with Li-derivatives of more complicated systems, e.g., ferrocene, is also described [229]. In the presence of TMEDA, hexafluorobenzene interacts with bis-Li-derivatives of ferrocene or dimethylaminomethylferrocene resulting in bis-pentafluorophenyl derivatives (Scheme 103) [229]. Similarly, a monolithium derivative (R = CH2N(CH3)2, X = H) with excess of hexafluorobenzene converts to mono-C6F5-derivative, and in the case of hexafluorobenzene deficiency 1,4-bis[2-(dimethylaminomethyl)ferrocene-1-yl]tetrafluorobenzene is formed [229].
Another type of reactions with Li-organic compounds is CAr-F bond activation due to the lithiation at ortho-position to the fluorine atom that promotes the cleavage of LiF and formation of a corresponding dehydrobenzyne capable of (4+2) cycloaddition. If bromine and hydrogen are present in ortho-position to the reacting fluorine then the lithiation occurs by replacing bromine with lithium. In the case of similar chloroderivatives H→Li substitution is favoured over Cl→Li. For chlordifluoroarenes with halogens in such positions that allow the formation of two different dehydrobenzynes, e.g., for 1-chloro-2,4-difluorobenzene the realized direction is that governed by the inductive effect of chlorine, that destabilizes positive charge on the ortho-carbon much more than that on the para-carbon (Scheme 104) [230].

That regioselectivity is confirmed by the preparation of corresponding fluorinated adducts of fluorinated dehydrobenzynes, produced from di-, tri- or pentafluorobromo- or –chloroarenes, by the Diels–Alder reactions with furan or N-methylpyrrol (Scheme 105) [230].
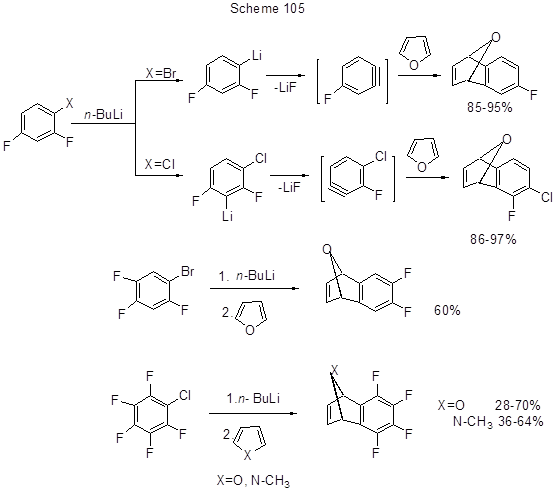
Step-by-step lithiation of 1,3,5-trifluorobenzene first using 3 or 6 equivalent amounts of t-BuLi continues by cascade of alternating LiF cleavage and t-BuLi adding to thus generated dehydrobenzynes, resulting in replacing all fluorines with tert-butyl groups (Scheme 106) [231].
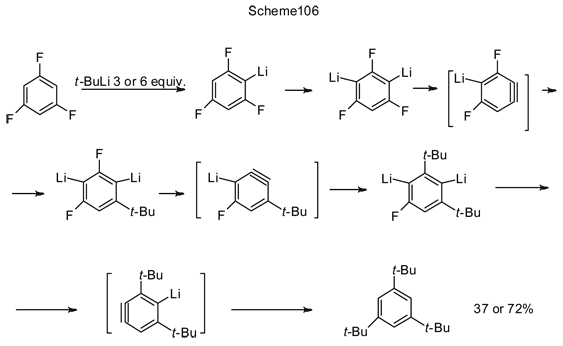
The above reactions with dehydrobenzene participation are usually conducted in ether medium, however, non-coordinating hydrocarbon solvents, e.g., pentane, hexane, heptane, dicyclohexane, petroleum-ether are also applicable for such conversion [232]. For example, 2,6-difluoroiodobenzene (1, Scheme 107) in petroleum-ether reacts with n-BuLi and further with cyclopentadiene, resulting in a mixture of two substances: 5-fluoro-1,4-dihydro-1,4-methanonaphthalene 3 and 5-(2,6-difluorophenyl)-1,4-dihydro-1,4-methanonaphthalene 4 [232].
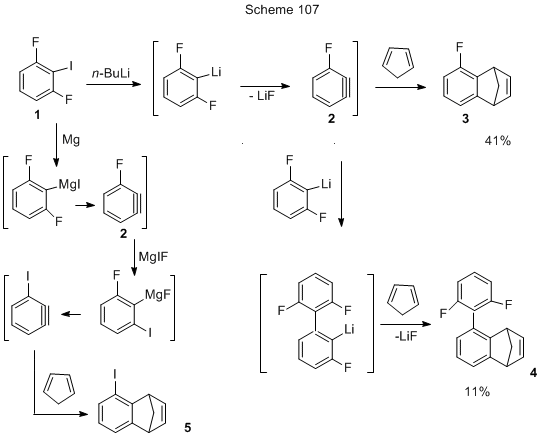
Dehydrobenzyne 2 might also be generated from iododerivative 1 and via Mg-organic compound, but in this case iododerivative 5 is produced in the same amount as 3, the total mixture yield being 41% [232].
1.3 Reactions with Mg-organic derivatives
Organomagnesium compounds were also intensively applied for the carbanion sources in nucleophilic substitution of fluorines in polyfluoroarenes with the intent of introduction of desirable C-groups [233]. Thus, under the action of CH3MgCl in the phenyl ring of 2-(2,6-difluorophenyl)-4,4-dimethyl-4,5-dihydro-1,3-oxazole fluorine is replaced with CH3-group, the yield being 96% [233]. In the similar manner methyl group was introduced into 1,3,4,5-tetrafluoroisophtalonitrile or 1,3,4,5-tetrafluoroisophtalic acid, during which introduction the replaced fluorine was in para-position to CN- or to COOH-group (the corresponding yields were 56% and 82%) [234]. Similar result was achieved when CH3MgBr is used in the reaction with the said nitrile. The reaction between tetrafluoroisophtalic acid and 2-methylphenylmagnsium chloride makes it possible to replace fluorine with 2-methylphenyl group [234]. In the course of preparation and investigation of liquid crystal structures the substitution of para-fluorine in pentafluorophenyl group of pentafluorophenyltrimethylsilylethyne with para-alkoxyphenyl group under the action of Mg-organic compound from para-alkoxybromobenzene was realised [235]. The action of 3,4-Cl2C6H3MgCl or 3-CNC6H4MgCl on 2,3,4,5,6-pentafluorobenzophenone results in the substitution of fluorines in positions 2 and 6 with the corresponding aryl moiety (the yields are 50% and 39%, respectively) [236]. The reaction between octafluoronaphtalene and C6F5MgBr resulted in 2-pentafluorophenylheptafluoronaphtalene with yield of 15% [237].
1.4 Effect of Si-organic derivatives in the presence of catalysts generating fluorine - ion.
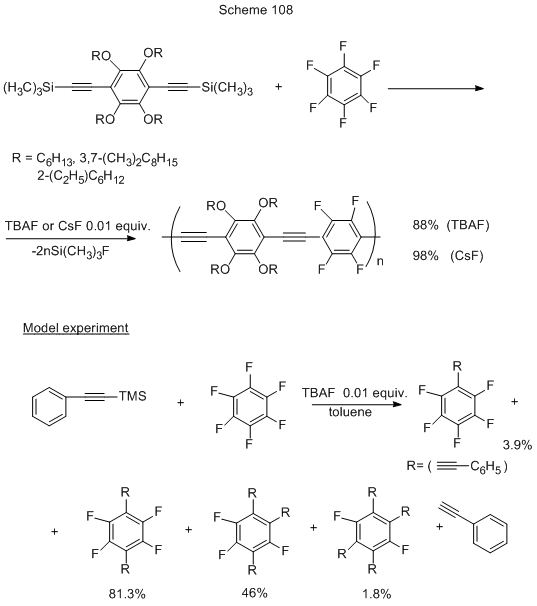
For the synthesis of poly(phenylene)ethinylenes with alternating aryl- and polyfluoroaryl links that may be of great interest because of their optoelectronic properties, a simple method was proposed, that is alternative to catalytic Sonogashira reaction, i.e. without the use of transition metal compounds, but leading to substances with high molecular weight, definite structure, purity, high yield. It is nucleophilic substitution of fluorine in polyfluoroarene under the action of Si-organic derivatives of alkyne-arenes in the presence of F- generating catalysts (CsF, TBAF, TMAF) (Scheme 108) [238]. Those reactions proceed at room temperature. The transformation occurs with pentafluoropyridine as well, but the excess of fluoride is then necessary. Otherwise they use it in catalytic amounts. In a model experiment (Scheme 108) the main product is that due to the substitution of two para-fluorines, even when the ratio hexafluorobenzene : trimethylsilyl derivative is 1:1, and that is due to the higher rate of the second fluorine substitution as compared to the first one.
For another example of Si-organic derivatives application in the presence of F- see Scheme 102 in Chapter 1.2 [228].
2. Transformations of CAr-F bond involving its activation.
Successful transformation of CAr-F bond to CAr-C in some cases requires its activation. Such activation can be achieved in various ways. One of those processes (the formation of dehydrobenzyne) was already mentioned above in chapter 1.2. The activation is also conducted by using microwave irradiation or various catalysts including those organic. Thus regiospecific polyfluoroarylation of ketene heterocyclic aminals proceeds successfully under microwave irradiation, as shown in Scheme 109 [239].
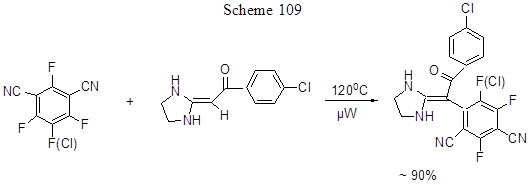
The nucleophilic aromatic substitution of fluorine at para-position to cyan-group in tetrafluoroisophtalonitrile, that of two para-fluorines in hexafluorobenzene or that of para-fluorine in pentafluorobenzonitrile, proceeds under the action of 2,3-dihydro-1,3-diisopropyl-4,5-dimethyl-imidazole-2-ylidene in the presence of BF3 etherate. The yields exceed 80% [240]. Tetrafluorophtalonitrile when heated with KI in DMF converts to 2,2’,5,5’,6,6’-hexafluoro-3,3’,4,4’-tetracyano-1,1’-biphenyl, the yield being 46% [241]. Both regio- and enantio-selective SNAr reactions with activated arenes and 1,3-dicarbonyl compounds proceed under the conditions of phase-transfer catalysis using a quaternary ammonium salt from Cinchona Alkaloids [242, 243]. Scheme 110 shows the reaction of nitropolyfluoroarene cycloalkylation under the action of either 2-carbethoxycyclopentanone, or N-substituted 3-carbethoxy-2-pyrrolidone at such conditions. The substitution of fluorine occurs only at its para-position regarding the nitro-group, and the more it activated the easier is the process. The process mechanism involves acidic proton abstraction from β-ketoester by a base, and the generation of an ambident nucleophile that reacts with a chiral quaternary ammonium salt to form a chiral ion pair ("chiral pocket"). The nucleophile alkaloid component contributes to its enantioselective nucleophilic approach to the aromatic substrate.
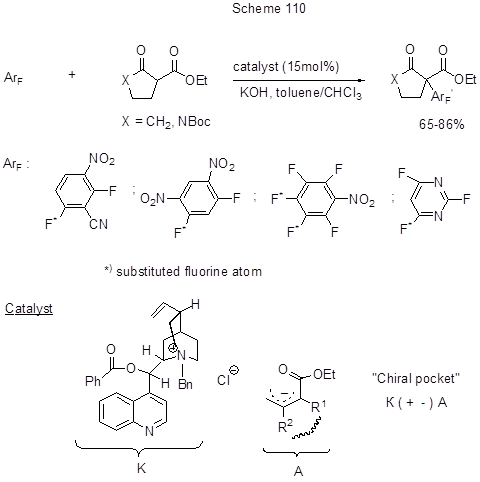
The transformation provides an interesting example of organocatalytic SNAr reaction. However, in recent time as it concerns CAr-F to CAr-C transformation considerably more importance and propagation were assigned to the techniques associated with oxidative addition of low-valent metals to C-F bond and further transformation. This is a wide range of cross-coupling reactions that involves such electrophiles as fluoroaromatic compounds, and such nucleophiles as organometallic compounds [Grignard reagents (the Kumada coupling), boron derivatives (the Suzuki reaction), Zn-reagents (the Negishi coupling), Sn-reagents (the Stille reaction)] in the presence of metal catalysts based on Pd, Ni, Pt, or Co. Different variants of those reactions for fluoroarenes, including polyfluoroarenes, have been reviewed elsewhere [213]. It turned out that C-F bond, the most strong of all CAr-Hal bonds, is not so easy to activate and to find transition metal complexes suitable for this. Thus, CAr-F bond is very selectively activated in monofluorobenzenes under the action of Ni-complexes [244], however, in the case of hexafluorobenzene those complexes are inactive [245]. However, the system Ni(cod)2 + PEt3 (or PBu3) was found for polyfluorohetarenes, that enabled the alkenylation of pentafluoropyridine and 2,3,5,6-tetrafluoropyridine via Stille reaction with Bu3SnCH=CH2 [246]. The reaction is regioselective and occurs at position 2 only in both cases. It is assumed that pre-coordination of pyridines at position 2 with Ni-centre resulting in the formation of Ni-complex is the determinant step of C-F bond activation (Scheme 111).
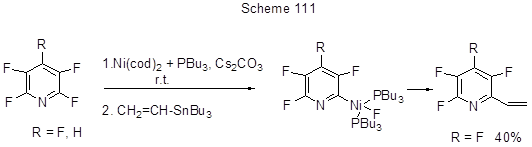
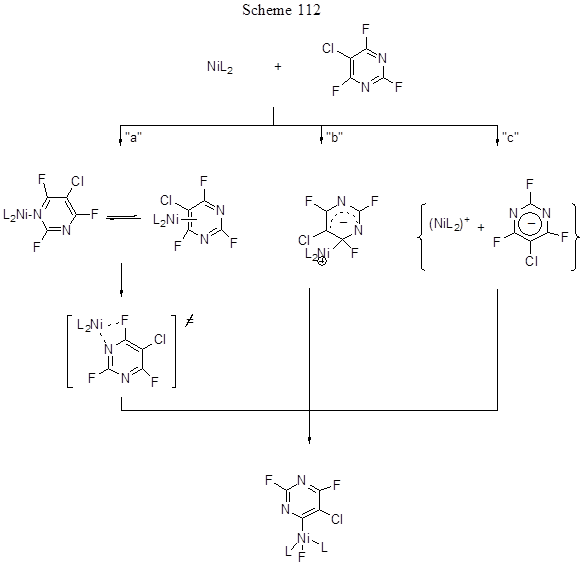
The by-products are 4-H-tetrafluoropyridine (from pentafluoropyridine by its reaction with trialkylphosphine) and 2,6-bis-vinyl derivative. It is interesting that CAr-H bond of 4-H-tetrafluoropyridine does not participate in the reaction. The same catalyst but with addition of various phosphines PR3 , here R=Cy, i-Pr, Ph, was applied for the arylation of 5-chloro-2,4,6-trifluoropyrimidine with ArB(OH)2 by Suzuki reaction, that occurs by 4,6 positions with yields from 40 to 90%. In this case the most suitable base is Cs2CO3 [244]. It is essential that the weaker CAr-Cl bond does not participate in it. The detailed analysis of transformation mechanism allowed assuming of three possible pathways for Ni-complex formation (Scheme 112), though none of them was proved experimentally. Pathway "a" includes the pyrimidine pre-coordination with nickel followed by its attachment to Ni-centre to be oxidizes. The alternative pathway “b” is nucleophilic substitution of fluorine initiated by NiL2 nucleophilic attack at the electrophilic position of the aromatic system, this being the rate-limiting step. Pathway «c» is electron transfer resulting in the formation of a contact ion pair.
Another catalytically active nickel system that is NHC-stabilized Ni-complex (here NHC is N-heterocyclic carbene) [Ni (i-Pr2Im)4 (cod)] 1 (Scheme 113) [245] which is a source of [Ni(i-Pr2Im)2], was successfully applied to a number of polyfluoroarenes, such as hexafluorobenzene, octafluorotoluene and decafluorobiphenyl and allowed to conduct arylation by Suzuki reaction. The reaction is of high regioselectivity and involves the substitution of fluorine in para-position (Scheme 113).
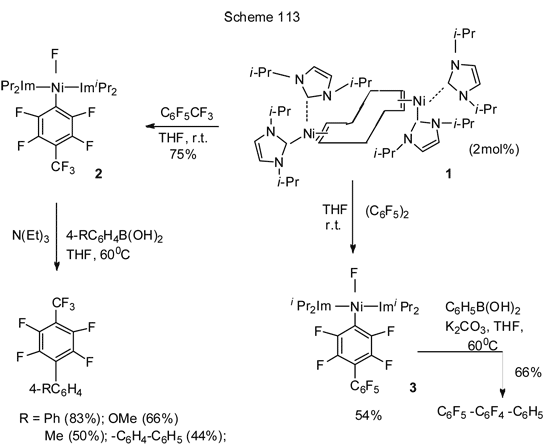
Catalytic system 1 is active in the Kumada cross-coupling with Grignard reagent, but its yield is generally lower. Thus, 4-(4'-methylphenyl)-heptafluorotoluene is produced by this reaction from octafluorotoluene and para-tolylmagnesiumbromide, but its yield is only 26% [245]. At the same time the Kumada phenylation of isomeric difluorobenzenes and 1,3,5-trifluorobenzenes with PhMgBr and Ni-catalyst involving triarylphosphine ligands is so active that all fluorines are replaced with phenyl groups [247]. The Kumada reaction of 2,4-difluorophenol with excess of n- C12H25MgBr in the presence of NiCl2 (diippbz) occurs selectively at the ortho-position to hydroxyl group, resulting in 75% yield of the corresponding 2-alkyl-4-fluorophenol [248]. Such regioselectivity is attributable to the effect of hydroxyl group and to the fact that the Ni-based catalytic system promotes ortho-cross-coupling of difluorophenol with alkyl-Grignard reagent, because at the transition state of Ni oxidative addition Lewis-acidic Mg facilitates the breakdown of C-F bond (Figure 114).
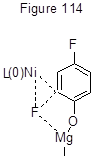
The selective replacement of ortho-fluorine in difluorosubstituted benzene derivatives with electron-donating substituents (OH, CH2OH, NH2, NHAc, NHBoc) by arylmagnesiumbromides is conducted as well with palladium catalyst PdCl2(PCy3)2. The yield of 2-aryl derivatives in the reactions of 2,4-difluorophenol exceeds 80% [249]. The use of [PdCl2(dppf)] in the reaction of 1,2-difluorobenzene with 4-CH3C6H4MgBr results in replacing of one fluorine with aryl group, while the use of Ni-catalyst NiCl2(dppp) results in a mixture of mono- and difluorosubstitution products, the share being nearly equal [5, p.56]. It is interesting to mark that the direction of the Stille reaction between pentafluoropyridine and tributylvinyl tin in the presence of palladium catalyst Pd[P(i-Pr)3]2 differs from that in presence of above-considered Ni-catalyst (this Chapter, Scheme 111). With the palladium-based catalyst a Pd-complex is formed at position 4, further converted to 4-vinyl-2,3,5,6-tetrafluoropyridine [250].
Among other transition-metal catalysts applied with fluoroarenes, one should mention TaCl5 that catalyzes the alkylation of hexafluorobenzene or pentafluorotoluene with 2-phenetylmagnesiumchloride resulting in the replacement of para-fluorine followed by the phenethyl group rearrangement (Scheme 115) [251].
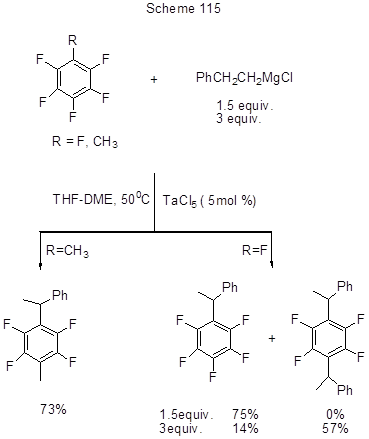
The use of cobalt catalyst in Kumada reaction with polyfluoroarenes is also of interest, because it avoids both the toxicity of nickel and expensiveness of palladium. Thus, two-step ortho,ortho-arylation of pentafluorobenzophenone occurs under the action of ArMgCl in the presence of Co(acac)2 and Cu(CN) . 2LiCl. Bu4NI and para-fluorostyrene perform as the reaction promoters (Scheme 116) [252]. During the reaction Mg-organic derivative reacts with copper salt to form, by way of transmetalation, a Cu-arene that is further cross-coupled with pentafluorobenzophenone to result in the final product. The study has successfully demonstrated the possibilities of involving Cu-organics into cross-coupling, the process being less investigated than the use of other organometallic compounds to form new C-C bonds in the series of arylfluorides.

Thermal decomposition of Cp2Zr(C6F5)2 zirconium complex in the presence of furan or durene results in the formation of Diels-Alder adducts, while the linear para-structured perfluoropolyphenylene oligomers F-[-2,3,5,6-C6F4-]nF, (n=2-13) are produced by the complex thermal decomposition in the presence of hexafluorobenzene or decafluorobiphenyl [253]. In the case of polyfluoroarenes the observed regularities are consistent with radical chain process initiated by impurities in those arenes and realized at the first, rapid step of the complex decomposition, as well as with the mechanism of intermediate formation of tetrafluorodehydrobenzyne, that is responsible for the formation of Diels-Alder reaction adducts in the case of furan and durene.
The activation of CAr-F bond in polyfluoroarenes resulting in polyfluoroarylzincorganic compounds occurs under the action of Zn in the presence of SnCl2 in DMF [254, 184]. By heating with CuCl2 thus formed Zn-organic derivatives convert to polyfluorobiaryls (Scheme 117) [254], and their reaction with allylchloride in CuCl/DMF system results in the formation of allyl derivatives [184]. Selective ortho-methylation of polyfluorinated arylimines with 2-5 fluorines in their aromatic rings, by CAr-F bonds, occurs under the action of dimethylzinc in the presence of Pt2Me4(SMe2)2 platinum catalyst (Scheme 118) [255]. The regioselectivity remains unchanged in the case of 2,6-difluoro-4-bromoderivative, despite the presence of weaker CAr-Br bond in the same molecule.
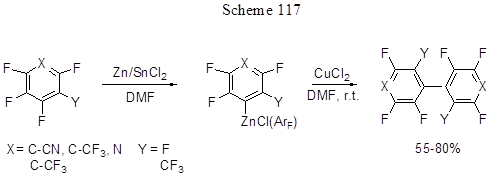
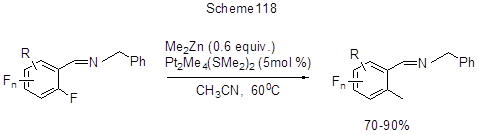
The activation of ortho-fluorine occurs for account of a cyclic Pt-complex formation with the participation of nitrogen and ortho-CAr-F bond. Under the action of Me2Zn transmetalation of the Pt-complex occurs, and the further reductive elimination results in the final product formation and Pt(II)-catalyst regeneration.
V. Transformation of other substituents in polyfluoroarene
The introduction of alkyl-, aryl- (hetaryl-) and unsaturated groups into polyfluoroarene is possible not only through the participation of CAr-H or CAr-Hal bonds, here Hal = Br, Cl, F, I, but also by way of the transformation of other substituents available in the molecule. Thus, the transformation of C=O bond in polyfluoroarylketones results in corresponding hydroxyalkyl derivatives (Scheme 119) [256-258].

Polyfluorinated stilbenes are produced by the decarbonylative Mizoroki-Heck cross-coupling of 3,5-difluorobenzoylchloride with substituted styrenes (Scheme 120) [259].
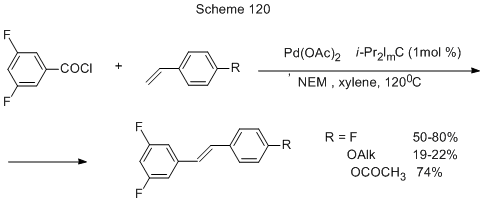
Similar decarboxylative hetarylation of 2,6-difluorobenzoic acid under the action of N-substituted indole allows the introduction of indole fragment instead of carboxyl group (Scheme 121) [260]. In this transformation Pd-catalyst activates position 3 of indole, while the silver salt contributes to the acid decarboxylation.
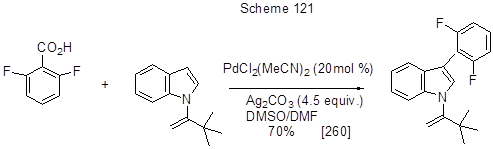
The decarboxylative cross-coupling of K-salts of di-, tri-, tetra-, or pentafluorobenzoic acids with various iodo- or bromoderivatives of benzene, pyridine or thiophene occurs only in the case of copper catalysis (CuI or CuI + Phen) (Scheme 122) [261].
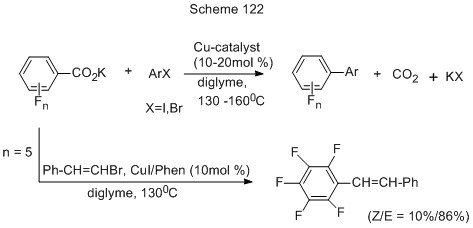
The technique does not use expensive and unstable organometallic catalysts, and CO2 is produced here instead of toxic metal halogenides. The method allows the production of polyfluorinated biaryls and adds those described in above chapters. Iodides react with high yields when CuI with/without ligands is applied, but for bromides the presence of a ligand is necessary. The used halogenated derivatives contained either electron-donating or electron-accepting substituents in ortho-, metha- or para-positions. Basing on the reactions of bromoderivatives an assumption was made that the reaction mechanism (Scheme 123) started with the interaction between the catalyst [iodo-copper complex (1)] and K-salt of an acid with the formation of Cu-salt of the acid (2), that is further decarboxylated via a 4-member cyclic transition state (TS 2-3) to give a new copper complex (3) involving a polyfluoroaromatic fragment. The copper complex interacts with arylbromide by oxidative addition via another transition state (TS 3-4), to form a new copper complex with polyfluoroaryl and aryl fragment (4), that further undergoes reductive elimination via a transition state (TS 4-5) resulting in the final biaryl.

When employing bromostyrenes in this reaction polyfluorinated stilbenes are produced [261]. 3,3',5,5'–Tetrafluorostilbene is also formed by the reaction of (3,5-difluorobenzyl)triphenylphosphonium bromide with 3,5-difluorobenzaldehyde (Scheme 124) [262].
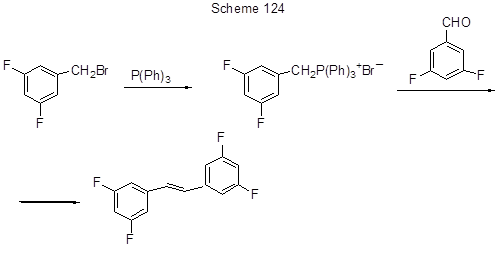
The vinyl fragment formation in position 5 of toluene 3,4-difluoro-5-hydroxyalkylderivative is due to dehydration (Scheme 125) [85], and benzylation of arenes that is a convenient way to diarylmethanes starting with benzyl alcohols (Scheme 126) [263 ].
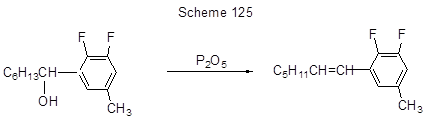
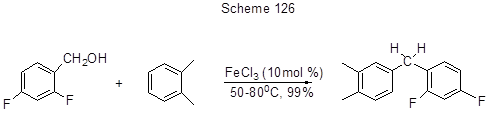
Two-step synthesis of 1-difluorophenyl-2-propanones from difluoroanilines and isopropylacetate by the Meerwein reaction was proposed in [264] (Scheme 127).
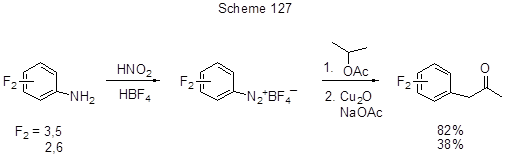
VI. CONCLUSION
The studies devoted to alkylation, arylation (hetarylation), alkenylation, and alkynylation of polyfluoroarenes demonstrate that for those substances, as well as for their non-fluorinated analogues, along with the classic methods, the more and more extended are those based on metal-complex catalysis, that with metals and ligands appropriately chosen makes it possible to introduce successfully desired groups into polyfluorinated arenes by replacing either hydrogen or any halogen, including fluorine. Such transformations often make a step of multi-step synthesis processes, and in many cases are used for the production of compounds with practicable useful properties. The development of those methods for polyfluoroarene series would certainly contribute to the extension in number of compounds containing polyfluoroaromatic groups, investigation of their chemical properties and search for their practical applications.
List of Abbreviations
1. acac — acetylacetonate
2. AIBN —a,a’ -azobisisobutyrodinitrile
3. BINAP — 2,2’ -bis(diphenylphosphino)-1,1’ - binaphthyl
4. (R)-BINAP — (R)-1,1’ - bis(diphenylphosphino)-2,2’ - binaphthyl
5. Boc — tert-butoxycarbonyl
6. COD — 1,5-cyclooctadiene
7. Cp — cyclopentadienyl
8. Cy — cyclohexyl
9. Cy2NH — N,N-dicyclohexylamine
10. Cyp — cyclopentyl
11. DABCO — 1,4-diazabicyclo[2,2,2]octane
12. davephos — (2-bicyclohexylphosphino)-2’ -(N,N-dimethylamino)biphenyle
13. dba — dibenzylideneacetone
14. Dt BPF — 1,1-bis(di-tert-butylphosphino)ferrocene
15. DiРPF — 1,1’ -bis(di-isopropylphosphino)ferrocene
16. DBU — 1,8-diazabicyclo[5.4.0]undec-7-ene
17. dppe — 1,2-bis(diphenylphosphino)ethane
18. dppf — 1,1’ -bis(diphenylphosphino)ferrocene
19. dppp — 1,3-bis(diphenylphosphino)propane
20. dppbz — 1,2-bis(diphenylphosphino)benzene
21. dii ppbz — 1,2-bis[(4-isopropylphenyl)phosphino]benzene
22. EPHP — 1-ethylpiperidine hypophosphite
23. L — ligand
24. LDA — lithium diisopropylamide
25. LiTMP — lithium tetramethylpiperidide
26. NHC — N-heterocyclic carbene
27. NEM — N-ethylmorpholine
28. OTf — trifluoromethanesulfonate
29. PCy3 - HBF4 — tricyclohexylphosphine tetrafluoroborate
30. Phen — 1,10-phenantroline
31. i-Pr2Im — 1,3-bis(2,6-diisopropylphenyl)imidazol-2-ylidene
32. i-Pr2ImC — 1,3-bis(2,6-diisopropylphenyl)imidazolinium chloride
33. Rh(acac) bis(C2H2) — Rhodium acetylacetonate/bis ethylene
34 PCy2-dmb — 2-(dicyclohexylphosphino)-2’ ,6’ -dimethoxybiphenyl
35. TBAB — tetrabutylammonium bromide
36. TBAF — tetrabutylammonium fluoride
37. TFA — trifluoroacetic acid
38. TMAF — tetramethylammonium fluoride
39. TMEDA — N,N,N’ ,N’ -tetramethylethylenediamine
40. Tf — trifluoromethanesulfonyl
41. tfp — tri(o-furyl)phosphine
42. TMP — 2,2,6,6-tetramethylpyperidine
43. TFSA — trifluoromethanesulphonic acid
44. Ts — tosyl (4-toluenesulphonyl)
References
[2] Morrison R., Bojd R. Organicheskaya khimiya., Mir, Moskva, (1974).
[3] Barton D., Ollis V.D. Obshchaya organicheskaya himiya, T.1., Khimiya, Moskva, (1981).
[4] Hassan J., Sevignon M., Gozzi C., Schulz E., Lemair M., Chem. Revs., (2002), 102, 1359-1470.
[5] Ed. Ackermann L. Modern Arylation Methods., John Wiley, Weinheim, (2009).
[6] Smit V.A., Dil'man A.D. Osnovy sovremennogo organicheskogo sinteza., BINOM, Laboratoriya znanij, Moskva, (2009).
[7] Eds. Meijere A., Diederich F. Metal-Catalyzed Cross-Coupling Reactions., John Wiley, Weinheim, (2004).
[8] Schlosser M. Organometallics in Synthesis, 2nd Ed., John Wiley, Chichester, (2002).
[9] Brooke G.M., J. Fluor. Chem., (1997), 86, 1-76.
[10] Filler R., Fiebig Jr. A.E., Mandal B.K., J. Fluor. Chem., (2000), 102, 185-188.
[11] Koda T., Yasntake M., Shinmyozu T., Org. Lett., (2001), 3, 1419.
[12] Tabart M., Picaut G., Desconclois J-F., Dutka-Maben S., Huet Y., Berthaud N., Bioorg. Med. Chem. Lett., (2001), 11, 919-922.
[13] Lundbeck H., Pat. WO 106534 (2009) (C.A., 2009, 151, 337044).
[14] Nakamura S., Sugimoto H., Ohwada T., J. Am. Chem. Soc., (2007), 129, 1724- 1732.
[15] Pat. US 211741 (2006) (C.A., 2006, 145, 377029).
[16] Pat. US 270682 (2006) (C.A., 2007, 146, 27833).
[17]Карпов В.М.,Меженкова Т.В.,Платонов В.Е.,Синяков В.Р.,Щёголева Л.Н.,Ж.орган.хим., (2002), 38, 1210-1217.
[18] Karpov V.M., Meshenkova T.V., Platonov V.E., Sinyakov V.R., J. Fluor. Chem., (2002), 117, 73-81. Karpov V.M., Meshenkova T.V., Platonov V.E., Sinyakov V.R.,Shchegoleva L.N., Russ. J. Org. Chem.,(2002), 38, 1158-1165 (English translation).
[19] Sinyakov V.R., Meshenkova T.V., Karpov V.M., Platonov V.E., Rybalova T.V., Gatilov Y.V., Russ. J. Org. Chem.,(2003),39, 837-842 (English translation).
[20] Zonov Ya.V., Karpov V.M., Platonov V.E., J. Fluor.Chem., (2007), 128, 1058- 1064.
[21] Sinyakov V.R., Meshenkova T.V., Karpov V.M., Platonov V.E., Russ. J. Org. Chem.,(2007),43, 1677-1685 (English translation).
[22] Karpov V.M., Mezhenkova T.V., Platonov V.E., Sinyakov V.R., J. Fluor. Chem., (2001), 107, 53-57.
[23] Sinyakov V.R., Meshenkova T.V., Karpov V.M., Platonov V.E., Rybalova T.V., Gatilov Y.V., Russ. J. Org. Chem.,(2006),42, 77-86 (English translation).
[24] a) Bonnefous C., Payne J.E., Roppe J., Zhuang H., Chen X., Severance D., Noble S.A., Smith N.D., Symons K.T., Nguyen P.M., Shiau A.K., Hassig C.A., Sablad M., Rosenkrants N., Zhang Y., Tadimeti S., Wang L., Rix P., Walsh J.P., Yazdani N., J. Med. Chem., (2009), 52, 3047-3062; b) Pat. WO 29592 (2009) (C.A., 2009, 150, 283051); c) Pat. WO 29617 (2009) (C.A., 2009, 150, 282846); d) Pat. WO 29625 (2009) (C.A., 2009, 150, 283049).
[25] Safina L.Yu., Selivanova G.A., Shteingarts V.D., Koltunov K.Yu., Tetrahedron Letters., (2009), 50, 5245-5247.
[26] Inglis S.R., Stojkoski C., Branson K.M., Cawthray J.F., Fritz D., Wiadrowski E., Pyke S.M., Booker G.W., J. Med. Chem., (2004), 47, 5405-5417.
[27] Breault G., Eyemann C.J., Geng B., Momingstar M., Reck F., Pat. WO 134378 (2006) [C.A. (2007), 146, 81779].
[28] Kato T., Saeki K., Kawazoe Y., Hakura A., Mutation Res., (1999), 439, 149- 158. [29] Laev S.S., Gurskaya L.Yu., Selivanova G.A., Beregovaya I.V., Shchegoleva L.N., Vasil’eva N.V., Shakirov M.M., Shteingarts V.D., Eur. J. Org. Chem., (2007), 306-316.
[30] Shuttleworth S.J., Lizarzabaru M.E., Chai A., Coward P., Bioorg. Med. Chem. Letters., (2004), 14, 3037-3042.
[31] Mustafin A.G., Gimadieva A.R., Khalilov I.N., Spirikhin L.N., Fatykhov A.A., Nurushev R.A., Abdrakhmanov I.B., Tolstikov G.A., Bull. Russ. Acad. Sci. Div. Chem.Sci., (1998), 47, 188-190 (English translation).
[32] Gataullin R.R., F. F. Minnigulov F.F., Kudashev A.R., Nurushev R.A., I. B. Abdrakhmanov I.B., Russ. J. Applied Chem., (2002), 75, 95-97 (English translation).
[33] Gataullin R.R., Kazhanova T.V., DavydovaV.A., IsmagilovaA.F., Zarudii F.S., Fatykhov A.A., SpirikhinL.V., I.B. AbdrakhmanovI.B., Russ. J. Chem. Pharm., (1999), 33, , 255-258.
[34] Song Yu., Zhu A., Lv J., Gong G., Xie J., Zhou J., Ye Y., Zhong X., Spectrochim. Acta Part A: Molecular and Biomol. Spectroscopy, (2009), 73, 96-100.
[35] Chackalamannil S., Doller D., Clasty M., Xia Y., Fagen K., Lin Y., Hsung-An Tsai, McPhail A.T., Tetrahedron Letters, (2000), 41, 4043-4047.
[36] Yoshimura H., Kikuchi K., Hibi Sh., Tagami K., Satoh T., Yamauchi T., Ishibahi A., Tai K., Hida T., Tokuhara N., Nagai M., J. Med. Chem., (2000), 43, 2929-2937.
[37] Pat. US 6110959 (2000) (C.A., 1997, 127, 278135); Pat. US 6355669 (2002) (C.A., 1999, 130, 306588); Pat. US 6258811 (2001) (C.A., 1998, 128, 270532).
[38] Pat. US 7045545 (2006) (C.A., 2000, 133, 135218).
[39] Kim B.H., Jeon I., Lee D.B., Park H.J., Jun Y.M., Baik W., J. Fluor. Chem., (2001), 109, 145-149. 106
[40] Yekta S., Krasnova L.B., Mariampillai B., Picard C.J., Chen G., Pandiaraju S., Yudin A.K., J. Fluor. Chem., (2004), 125, 517-525; Pat. WO 51899 (2005) (C.A., 2005, 143, 27355).
[41] Pat. WO 60160 (2009) (C.A., 2009, 150, 313452).
[42] Pat. US 78249 (2003) (C.A., 2001, 134, 86149).
[43] Lafrance M., Rowley Ch.N., Woo T.K., Fagnou K., J. Am. Chem. Soc., (2006), 128, 8754-8756.
[44] Lafrance M., Shore D., Fagnou K., Org. Letters, (2006), 8, 5097-5100.
[45] Youbert N., Urban M., Pohl R., Hocek M., Synthesis, (2008), 1918-1932.
[46] Litvinas N.D., Brodsky B.H., DuBois J., Angew. Chem., Int. Ed.Engl., (2009), 48, 4513-4516.
[47] Zhao D., Wang W., Lian S., Yang F., Lan J., You J., Chemistry – A Europ. J., (2009), 15, 1337-1340.
[48] Itahara T., J. Org. Chem., (1985), 50, 5546-5550.
[49] Ahn J.H., Cho S.Y., Ha J-D., Chu S.Y., Jung S.H., Jung Y.S., Baek J.J., Choi I.K., Shin E.Y., Kang S.L., Kim S.S., Cheon H.G., Yang S.D., Choi J.K., Bioorg. Med. Chem. Letters, (2002), 12, 1941-1946.
[50] Rauf W., Thompson A.L., Brown J.M., Chem. Commun., (2009), 3874-3876.
[51] Laha J.K., Petron Ph., Cuny G.D., J. Org. Chem., (2009), 74, 3152-3155.
[52] Chernyak N., Gevorgyan V., J. Am. Chem. Soc., (2008), 130, 5636-5637; Advanced Synth. and Catalysis, (2009), 351, 1101-1114.
[53] Wei Y., Kan J., Wang M., Li W., Hong M., Org. Letters, (2009), 11, 3346- 3349.
[54] Demchuk O.M., Pietrusiewicz K.M., Synlett, (2009), 18, 1149-1153.
[55] Pat. US 42896 (2009) (C.A., 2009, 150, 237419).
[56] Saito A., Oda S., Fukaya H., Hanzawa Yu., J.Org. Chem., (2009), 74, 1517- 1524.
[57] Nakao Y., Kashihara N., Kanyiva K.S., Hiyama T., J. Am. Chem. Soc., (2008), 130, 16170-16171.
[58] Pat. US 6211375 (2001) (C.A., 1997, 127, 331407).
[59] Pat. US 6136823 (2000) (C.A., 1998, 129, 41087).
[60] Pat. US 6235764 (2001) (C.A., 2000, 132, 22961).
[61] Pat. US 6348624 (2002) (C.A., 1999, 131, 73434); Pat. US 6329391 (2001) (C.A., 2002, 136, 20031).
[62] Pat. EP 1223169 (2002) (C.A., 1996, 125, 142540).
[63] Pat. US 114666 (2003) (C.A., 2003, 138, 55976).
[64] Chodakowski J., Klis’ T., Servatowski J., Tetrahedron Letters, (2005), 46, 1963-1965.
[65] Anquetin G., Greiner J., Vierling P., Tetrahedron, (2005), 61, 8394-8404.
[66] Omura K., Uchida T., Irie R., Katsuki T., Chem. Commun., (2004), 2060- 2061.
[67] Leroux F., Hutsehenreuter T.U., Charriere C., Scopelliti R., Hartman R.W., Helv. Chim. Acta, (2003), 86, 2671-2686.
[68] Inagaki H., Tsuruoka H., Hornsby M., Lesley S.A., Spraggon G., Ellman J.A., J. Med. Chem., (2007), 50, 2693-2699.
[69] Bashore C.G., Vetelino M.G., Wirtz M.C., Brooks P.R., Frost H.N., McDermort R.E., Whritenour D.C., Ragan J.A., Rutherford J.L., Makowski T.W., Brenek S. J., Coe J.W., Org. Letters, (2006), 8, 5947-5950.
[70] Becht J.M., Ngouela S., Wagner A., Mioskowski Ch., Terahedron, (2004), 60, 6853-6858.
[71] Leroux F.R., Bonnafoux L., Heiss C., Colobert F., Lanfranchi D.A., Advanced Synth. and Catalysis, (2007), 349, 2705-2713.
[72] Milne J.E., Buchwald S.L., J. Am. Chem. Soc., (2004), 126, 13028-13032.
[73] Heis C., Leroux F., Schlosser M., Eur. J. Org. Chem., (2005), 5242-5247.
[74] Pat. US 6388146 (2002) (C.A., 1999, 131, 163453).
[75] Scott J.P., Brower S.E., Davis A.J., Brands K.M.J., Synlett, (2004), 1646-1648.
[76] Davies A.J., Scott J.P., Bishop B.C., Brands K.M., Brewer S.E., DaSilva J.O., Dormer P.G., Dolling U-H., Gibb A.D., Hammond D.C., Lieberman D.D., Palucki M., Payack J.T., J. Org. Chem., (2007), 72, 4864-4871.
[77] Pat. US 42851 (2009) (C.A., 2007, 147, 234875).
[78] Nishida J., Fujiwara Yu., Yamashita Y., Org. Letters, (2009), 11, 1813-1816.
[79] Pat. US 131482 (2009) (C.A., 2009, 150, 563826).
[80] Chen Sh., Huby N.J.S., Kong N., Moliterni J.A., Jonh A., Morales O.J., Pat. US 170920 (2009) (C.A., 2009, 151, 123990).
[81] Keum H.-W., Roh S.D., Do Y.-S., Lee J.-H., Kim Y.-B., Mol. Crystals and Liquid Crystals, (2005), 439, 189-199.
[82] Bremer M., Lietrau L., New J. Chem., (2005), 29, 72-74.
[83] Pat. US 247585 (2007) (C.A., 2007, 147, 494408).
[84] a) Glendenning M.E., Goodby J.W., Hird M., Toyne K.J., J. Chem. Soc., P2, (2000), 27-34; b) J. Chem. Soc., P2 (1999), 481-491.
[85] Cammidge A.N., Crepy K.V.L., J. Org. Chem., (2003), 68, 6832-6835. [86] Popov I., Do H-Q., Daugulis O., J. Org. Chem., (2009), 74, 8309-8313.
[87] Coe P.L., Rees A.J., J. Fluor. Chem., (2000), 101, 45-60.
[88] Do H.-Qu., Daugulis O., J. Am. Chem. Soc., (2008), 130, 1128-1129.
[89] Do H.-Qu., Khaw R.M.K., Daugulis O., J. Am. Chem. Soc., (2008), 130, 15185-15192.
[90] Pat. US 76266 (2009) (C.A., 2009, 150, 329777).
[91] Do H.-Qu., Daugulis O., Chem. Commun., (2009), 6433-6435.
[92] Matsuyama N., Kitahara M., Hirano K., Satoh T., Miura M., Org. Letters, (2010), 12, 2358-2361.
[93] Susuki A., J. Organomet. Chem., (1999), 576, 147-168.
[94] Molander G.A., Biolatto B., J. Org. Chem., (2003), 68, 4302-4314.
[95] Kotsikorou E., Song Y., Chan J.M.W., Faelens S., Tovian Z., Broderick E., Bakalara N., Docampo R., Oldfield E., J. Med. Chem., (2005), 48, 6128-6139.
[96] Song Y., Oldfield E., Lin F.-Y., Yin F, Mikkamala D., Dushyant C., Cao R., Hensler M., Nizet V., Poveda C-A.R., Pacanowska D.G., Wang H., Morita C.T., J. Med. Chem., (2009), 52, 976-988.
[97] Kumar M., Kaur K., Sinha S., Gupta S., Palle V., Chugh A., Pat US 12116 (2009) (C.A., 2007, 146, 162865).
[98] Feuerstein M., Berthiol F., Doucet H., Santelli M., Synlett, (2002), 1807-1810.
[99] Kulmaelae T., Kuuloja N., Franzen R.X., Rissanen R.K., Europ. J. Org. Chem., (2008), 4019-4024.
[100] Guo M., Zhang Q., Tetrahedron Letters, (2009), 50, 1965-1968.
[101] Duplantier A.J., Kraus K.G., Lu J., Noha M., Rogers B.N., Zhang L., Efremov I., Candler J., O’ Sillivan T.J., Ganong A.H., Hanks A.N., Lazzaro Jr. J.T., McCarthy S.A., Siuciak J.A., Doran A.C., Haas J.A., Spracklin D.K., Bioorg. Med. Chem. Letters, (2009), 19, 2524-2529.
[102] Pat. EP 1842854 (2007) (C.A., 2007, 147, 427511); Pat. WO 148829 (2008) (C.A., 2009, 150, 44027); Pat. US 200920 (2009) (C.A., 2007, 146, 430964).
[103] Pat. WO 11447 (2009) (C.A., 2009, 150, 121792).
[104] Pat. US 163508 (2009) (C.A., 2009, 150, 447978). 109
[105] Pat. WO 112854 (2009) (C.A., 2009, 151, 392217).
[106] Pat. WO 17664 (2009) (C.A., 2009, 150, 191528); Pat. WO 10150 (2010) (C.A., 2010, 152, 215298).
[107] Pat. US 203705 (2009) (C.A., 2009, 151, 245649).
[108] Pat. WO 155388 (2009) (C.A., 2010, 152, 97501); Pat. WO 155389 (2009) (C.A., 2010, 152, 97472).
[109] Pat. WO 102460 (2009) (C.A., 2009, 151, 289162).
[110] Pat. WO 153285 (2009) (C.A., 2010, 152, 97447).
[111] Ce G., Guo H., He J., Wang F., Zou D., J. Organomet. Chem., (2009), 694, 3050-3057.
[112] Lumeras W., Caturla F., Vidal L., Esteve C., Vidal B., Balaque C., Orellana A., Godessard N., Dominguez M., Roca R., Huerta J.M., J. Med. Chem., (2009), 52, 5531-5545.
[113] Imbriglio J.E., Chang S., Liang R., Raghavan S., Schmidt D., Smenton A., Tria S., Schrader T.O., Jung J.K., Esser C., Taggart A.K.P., Cheng K., Carballo-Jane E., Waters M.G., Tata J.P., Colletti S.L., Bioorg. Med. Chem. Letters, (2009), 19, 2121-2124.
[114] Matin A., Gavandl N., Kim M.S., Yang N.X., Salam N.K., Hanzahan J-R., Roubin R.H., Hibbs D.E., J. Med. Chem., (2009), 52, 6835-6850.
[115] Pat. WO 26657 (2009) (C.A., 2009, 150, 306367).
[116] Hagdar S.N., Comery T.A., Dunlop J., Ghiron Ch., Bettinetti L., Bochmann H., LaPosa S., Micco I., Pollastrini M., Quinn J., Roncarati R., Scali C., Valacchi M., Varrone M., Zanaletti R., Bioorg. Med. Chem., (2009), 17, 5247-5258.
[117] Lee S., Yi K.Y., Youn S.J., Lee B.A., Yoo S., Bioorg. Med. Chem. Letters, (2009), 19, 1329-1331.
[118] Pat. WO 52341 (2009) (C.A., 2009, 150, 472421).
[119] Blake T.D., Hamper B.C., Huang W., James R., Moon J.B., Neel B.E., Olson K.L., Pelc M.J., Schneitzer B.A., Thorarensen A., Trujillo J.I., Turner S.R., Pat. US 146569 (2008) (C.A., 2008, 149, 79492).
[120] Pat. US 170907 (2009) (C.A., 2007, 146, 62449).
[121] Burns M.J., Fairlamb I.J.S., Kapdi A.R., Sehnal P., Taylor R.J.K., Org. Letters, (2007), 9 , 5397-5400; Fairlamb I.J.S., Sehnal P., Taylor R.J.K., Synthesis, (2009), 508-510.
[122] Pat. EP 2123623 (2009) (C.A., 2008, 149, 235600). 110
[123] a) Pat WO 120789 (2009) (C.A., 2009, 151, 403339); b) Pat. EP 2116522 (2009) (C.A., 2008, 149, 343403).
[124] Li G., Stamford A.W., Huang Y., Cheng K.Ch., Cook J., Farley C., Gao J., Ghibaudi L., Greenlee W.J., Guzzi M., van Heek M., Hwa J.J., Kelly J., Mullins D., Parker E.M., Wainhaus S., Zhang X., Bioorg. Med. Chem. Letters, (2008), 18, 1146- 1150.
[125] Bouillot A.M., Dodic N., Gellibert F.J., Mirguet O., Pat. WO 15652 (2010) (C.A., 2010, 152, 238948).
[126] Demir A.S., Findik H., Saygili N., Subari N.T., Tetrahedron, (2010), 66, 1308-1312.
[127] Dong Ch.Ch., Styring P., Goodby J.W., Chan L.K.M., J. Mater. Chem., (1999), 9, 1669-1678.
[128] Yelamaggad C.V., Mathews M., Hizemath U.S., Rao D.S., Prasad S.K., Tetrahedron Letters, (2005), 46, 2623-2626.
[129] Deeg O., Bauerle P., Org. and Biomol. Chem., (2003), 1, 1609-1624.
[130] Pat. WO 52722 (2006) (C.A., 2006, 144, 488936).
[131] Tomson S.A., Banker P., Bickett D.M., Boucheron J.A., Carter H.L., Clancy D.C., Cooper J.P., Dickerson S.H., Garrido D.M., Nolte R.T., Peat A.J., Sheckler L.R., Sparks S.M., Tavares F.X., Wang L., Wang T.Y., Weiel J.E., Bioorg. Med. Chem. Letters, (2009), 19, 1177-1182.
[132] Wood W.J.L., Patterson A.W., Tsuruoka H., Jain R.K., Ellman J.A., J. Am. Chem. Soc., (2005), 127, 15521-15527.
[133] Pat. WO 77385 (2009) (C.A., 2009, 151, 77775).
[134] Liu P., Zhou L., Li X., He R., J. Organomet. Chem., (2009), 694, 2290-2294.
[135] Bey E., Marchais-Oberwinkler S., Negri M., Kruchten P., Oster A., Kllin T., Spadaro A., Werth R., Frotscher M., Birk B., Hartmann R.W., J. Med. Chem., (2009), 52, 6724-6743.
[136] Pat. WO 45383 (2009) (C.A., 2009, 150, 396692).
[137] Pat. US 258866 (2009) (C.A., 2008, 148, 17746).
[138] Jaroch S., Hölscher P., Rehwinkel H., Sülzle D., Burton G., Hillmann M., McDonald F.M., Bioorg. Med. Chem. Letters, (2002), 12, 2561-2564; Jaroch S., Rehwinkel H., Hölscher P., Sülzle D., Burton G., Hillmann M., McDonald F.M., Miklautz H, Bioorg. Med. Chem. Letters, (2004), 14, 743-746.
[139] Zhou L., Du X., He R., Ci Z., Bao M., Tetrahedron Letters, (2009), 50, 406- 408.
[140] Stencel L.M., Kormos C.M., Avery K.S., Leedbeater N.E., Org. Biomol. Chem., (2009), 7, 2452-2457.
[141] Pat. US 239748 (2009) (C.A., 2007, 146, 121670).
[142] Pat. EP 2011788 (2009) (C.A., 2007, 147, 502372).
[143] Barberousse V., Bondoux M., Tomas D., Peyrou V., Pat. US 293768 (2008) (C.A., 2006, 145, 377529); Bondoux M., Mignon L., Ou K., Renaut P., Tomas D., Barberousse V., Tetrahedron Letters, (2009), 50, 3872-3876.
[144] Eidenschink R., Kretzchmann H., Pat. US 131689 (2009) (C.A., 2007, 147, 200299).
[145] Pat. WO 91884 (2009) (C.A., 2009, 151, 208832).
[146] Pat. US 29935 (2010) (C.A., 2008, 148, 403569).
[147] Lugo B., Allbutt B., Beaumont D., Butt U., James A.R., Synlett, (2009), 675- 680.
[148] Taugerbeck A., Montenegro E., Manabe A., Plach H., Pat US 247620 (2009) (C.A., 2007, 147, 177293).
[149] Hird M., Goodby J.W., Gough N., Toyne K.J., J. Mater. Chem., (2001), 11, 2732-2742.
[150] Raghavan S., Schmidt D.R., Darby R., Steven S.L., Abi- Smenton A.L., Pat. US 62269 (2009) (C.A., 2007, 147, 277179).
[151] Korenaga T., Kosaki T., Fukumura R., Ema T., Sakai T., Org. Letters, (2005), 7, 4915-4917.
[152] a) Frohn H-J., Adonin N. Yu., Bardin V.V., Starichenko V.F., J. Fluor. Chem., (2003), 122, 195-199; b) Tetrahedron Letters, (2002), 43, 8111-8114; c) J. Fluor. Chem., (2002), 117, 115-120.
[153] Pat. WO 109743 (2009) (C.A., 2009, 151, 337021).
[154] Levi M.D., Gofer Y., Cherkinsky M., Birsa M.L., Aurbach D., Berlin A., Phys. Chem. Chem. Phys., (2003), 5, 2886-2893.
[155] Mosrin M., Knochel P., Org. Letters, (2009), 11, 1837-1840.
[156] Pat. EP 987238 (2000) (C.A., 1998, 129, 223634).
[157] Wunderlich S.H., Knochel P., Angew. Chem., Int. Ed.Engl., (2009), 48, 1501- 1504.
[158] Bromidge S.M., Dabts S., Davies D.T., Davies S., Duckworth D.M., Forbes I.T., Gaster L.M., Ham P., Jones G.E., King F.D., Mulholand K.R., Saunders D.V., Wyman P.A., Blaney F.E., Clarke S.E., Blackburn T.P., Holland V., Kennett G.A., Lightower S., Middlemiss D.N., Trail B., Rilly G.J., Wood M.D., J. Med. Chem., (2000), 43, 1123-1134. [158a] Pat. WO 14268 (2009), (C.A., (2009), 150, 213983).
[159] a) Hatakeyama T., Hashimoto S., Ishizuka K., Nakamura M., J. Am. Chem. Soc., (2009), 131, 11949-11963; b) Hatakeyama T., Nakamura M., J. Am. Chem. Soc., (2007), 129, 9844-9845.
[160] Mayer M., Czaplik W.M., Jacobi von Wangelin A., Synlett, (2009), 2919- 2923.
[161] Ok H.O., Reigle L.B., Candelore M.R., Cascieri M.A., Colwell L.F., Deng L., Feeney W.P., Forrest M.J., Hom G.J., MacIntyre D.E., Stader C.D., Tota L., Wang P., Wyvratt M.J., Fisher M.H., Weber A.E., Bioorg. Med. Chem. Letters, (2000), 10, 1531-1534.
[162] Hatakeyama T., Kondo Y., Fujiwara Yu-J., Takaya H., Nakamura M., Ito Sh., Nakamura E., Chem. Commun., (2009), 1216-1218.
[163] Van Huis Ch.A., Casimiro-Garcia A., Bigge C.F., Cody W.L., Dudley D.A., Filipski K-J., Heemstra R.J., Kohrt J.T., Leadley Jr. R.J., Narasimhan L.S., McClanahan T., Mochalkin I., Pamment M., Thomas P.T., Sahasrabudhe V., Schaum R.P., Edmunds J.J., J. Bioorg. Med. Chem., (2009), 17, 2501-2511.
[164] Pat. WO 13126 (2009) (C.A., 2009, 150, 191522); Orsini P., Menichin-Cheri M., Vanotti E., Panzeri A., Tetrahedron Letters, (2009), 50, 3098-3100.
[165] Pat. WO 70869 (2009) (C.A., 2009, 151, 33423).
[166] Davies A.J., Scott J.P., Bishop B.C., Brands K.M., Brewer S.E., DaSilva J.O., Dormer P.G., Dolling U-H., Gibb A.D., Hammond D.C., Lieberman D.D., Palucki M., Payack J.T., J. Org. Chem., (2007), 72, 4864-4871.
[167] Pat. WO 140128 (2009) (C.A., 2009, 151, 571119).
[168] Abarbri M., Dehmel F., Knochel P., Tetrahedron Letters, (1999), 40, 7449- 7454.
[169]. ZAO NPO «Pim-Invest». Sintezy ftororganicheskih soedinenij., Izd.«Trovant», Moskva, (2005), c. 123, 124.
[170] Scheuermann G.M., Steurer P., Muelhaupt R., Rumi L., Bannwarth W., J. Am. Chem. Soc., (2009), 131, 8262-8270.
[171] Zhang X., Mu F., Robinson B., Wang P., Tetrahedron Letters, (2010), 51, 600-601.
[172] Audia J.E., Mergott D.J., Sheehan S.M., Watson B.M., Pat. US 275566 (2009) (C.A., 2009, 151, 528778).
[173] Pat. WO 103007 (2009) (C.A., 2009, 151, 288987).
[174] Gordeev M.F., Pat US 48305 (2009) (C.A., 2009, 150, 214368).
[175] Pulici M., Zuccotto F., Badari A., Nuvoloni S., Cervi G., Traquandi G., Biondaro S., Trifiro P., Marchionni C., Modugno M., Pat. WO 10154 (2010) (C.A., (2010), 152, 215278).
[176] Liu J., Jian T., Guo L., Atanasova T., Nargund R.P., Tetrahedron Letters, (2009), 50. 5228-5230.
[177] Heiss C., Schlosser M., Eur. J. Org. Chem., (2003), 447-451.
[178] Morgan B.P., Galdamez G.A., Gilliard Jr. R.J., Smith R.C., Dalton Trans., (2009), 2020-2028.
[179] a) Organ M.G., Calimsiz S., Sayah M., Hoi K.H., Lough A.J., Angew. Chem., Int. Ed.Engl., (2009), 48, 2383-2387; b) Altenhoft G., Goddard R., Lehmann Ch.W., Glorius F., J. Am. Chem. Soc., (2004), 126, 15195-15201.
[180] Sakamoto Y., Suzuki T., Miura A., Fujikawa H., Tokito S., Taga Y., J. Am. Chem. Soc., (2000), 122, 1832-1833.
[181] Yudin A.K., Martyn L.J.P., Pandiaraju S., Zheng J., Lough A., Org. Letters, (2000), 2, 41-44.
[182] Pat. WO 111299 (2009) (C.A., 2009, 151, 358919).
[183] Burton D.J., Hartgraves G.A., J. Fluor.Chem., (2009), 130, 254-258.
[184] Vinigradov A.S., Krasnov V.I., Platonov V.E., Russ. J. Org. Chem., (2008), 44, 95-102 (English translation).
[185] Hori A., Kataoka H., Akacaka A., Okano T., Fujita M., J. Polymer Sci., part A: Polymer Chem., (2003), 41, 3478-3485.
[186] Facchetti A., Yoon M-H., Stern Ch.L., Katz H.E., Marks T.J., Angew. Chem., Int. Ed. Engl., (2003), 42, 3900-3903. 114
[187] Cho D.M., Parkin S.R., Watson M.D., Org. Letters, (2005), 7, 1067-1068.
[188] Blass B.E., Huang C.T., Kawamoto R.M., Li M., Liu S., Portlock D.E., Rennells W.M., Simmons M., Bioorg. Med. Chem. Letters, (2000), 10, 1543-1546.
[189] Ragni R., Orselli E., Kottas G.S., Omar O.H., Babudri F., Pedone A., Naso F., Farinola G., DeCola L., Chemistry – A Europ. J., (2009), 15, 136-148.
[190] Nagao I., Shimizu M., Hiyama T., Angew. Chem., Int. Ed. Engl., (2009), 48, 7573-7576.
[191] Frattarelli D., Ratner M.A., Marks T.J., Schiavo M., Facchetti A., J. Am. Chem. Soc., (2009), 131, 12595-12612.
[192] Pat. WO 106577 (2009) (C.A., 2009, 151, 313566).
[193] Pat. EP 1674452 (2006) (C.A., 2005, 142, 355051); Pat. EP 1780197 (2007) (yield 83%) (C.A., 2006, 144, 191974); Tecle H., Shao J., Li Y., Kuthe M., Kazmirski S., Penzotti J., Ding Y-H, Moshinsky D., Coli R., Jhawar N., Bora E., JaquesO’ Hagan S., Wu J., Ohren J., Bioorg. Med. Chem. Letters, (2009), 19, 226-229 (yield 65%).
[194] Wang C.H., Alluri S., Ganguly A.K., Tetrahedron Letters, (2009), 50, 1879- 1881.
[195] Matsuda Sh., Takahashi M., Monguchi D., Mori A., Synlett, (2009), 1941- 1944.
[196] Huang Ch., Gevorgyan V., J. Am. Chem. Soc., (2009), 131, 10844-10845.
[197] Geramita K., McBee J., Tilley T.D., J. Org. Chem., (2009), 74, 820-829.
[198] Marchetti F., Pampaloni G., Passarelli V., Masi F., Sommazzi A., Spera S., J. Fluor. Chem., (2009), 130, 341-347.
[199] Pat. WO 117097 (2009) (C.A., 2009, 151, 403303).
[200] Baumann K., Flohr A., Goetschi E., Jacobsen H., Jolidon S., Luebbers T., Pat. US 181965 (2009) (C.A., 2009, 151, 148313).
[201] Oswald C.L., Peterson J.A., Lam H.W., Org. Letters, (2009), 11, 4504-4507.
[202] Mariaca R., Labat G., Behrnd N-R., Bonin M., Helbling F., Eggli P., Couderc G., Hulliger J., Neels A., Stoeckli-Evans H., J. Fluor. Chem., (2009), 130, 175-196.
[203] Jayanth T.T., Zhang L., Johnson T.S., Malinakova H.C., Org. Letters, (2009), 11, 815-818.
[204] Takayuki T., Yuhei Y., Masashi K., Toshiaki M., Org. Letters. (2009), 11, 1043-1045.
[205] Martin R.E., Wytko J.A., Diederich F., Helv. Chim. Acta, (1999), 82, 1470- 1479.
[206] Rosenblum S.B., Huynh T., Afonso A., Davis Jr. H.R., Tetrahedron, (2000), 56, 5735-5742.
[207] Pat. US 42841 (2009) (C.A., 2009, 150, 168179).
[208] Lledo A., Restorp P., Rebeck Jr. J., J. Am. Chem. Soc., (2009), 131, 2440- 2444.
[209] a) Isaac M., Slassi A., Silva K.D., Xin T., Tetrahedron Letters, (2001), 42, 2957-2960; b) Ying M., Smentek M.G., Day C.S., Welker M.E., Ma R., Torti S.V., Letters in Org. Chem., (2009), 6, 242-251.
[210] Matsnev A., Noritake S., Nomura Y., Tokunaga E., Nakamura S., Shibata N., Angew. Chem., Int. Ed. Engl., (2010), 49, 572-576.
[211]. Biffis A., Scattolin E., Ravasio N., Zaccheria F., Tetrahedron Letters, (2007), 48, 8761-8764; Luque R., MacQuarrie D.J., Org. Biomol. Chem., (2009), 7, 1627- 1632; Johnson S.A., Liu F-Q., Suh M.C., Zuercher S, Haufe M., Mao S.S.H., Tilley T.D., J. Am. Chem. Soc., (2003), 125, 4199-4211; Zeidan T.A., Kovalenko S.V., Monoharan M., Clark R.J., Ghiviriga J., Alabugin I.V., J. Am. Chem. Soc., (2005), 127, 4270-4285; Smith C.E., Smith P.S., Thomas R.L., Robins E.G., Collings J.C., Dai Ch., Scott A.J., Borwick S., Batsanov A.S., Watt S., Clark C.J., VineyCh., Howard J.A.K., Clegg W., Marder T.B., J. Mater. Chem., (2004), 14, 413-420.
[212] a) Ojima I. Fluorine in Medicinal Chemistry and Chemical Biology, J. Wiley Publication , Willy-Blackwell, (2009), p. 299, 301; b) Benmansour H., Chambers R.D., Sandford G., Yufit D.S., Hovard J.A.K., Arkivoc, (2007), (XI), 46-55.
[213] Amii H., Uneyama K., Chem. Revs., (2009), 109, 2119-2183.
[214] Deck P.A., Kroll C.E., Hollis W.G., Fronczek F.R., J. Organomet. Chem., (2001), 637-639, 107-115.
[215] Deck P.A., McCauley B., Slebodnick C., J. Organomet. Chem., (2006), 691, 1973-1983.
[216] Gurjar M., Reddy D.S., Murugaiah A., Murugaiah S., Synthesis, (2000), 1659- 1661.
[217] [212a, p. 307].
[218] Sasaki Sh., Tanabe Y., Yoshifuji M., Bull. Chem. Soc. Japan, (1999), 72, 563- 572.
[219] Morrison D.J., Trefz T.K., Piers W.E., McDonald R., Parvez M., J. Org. Chem., (2005), 70, 5309-5312.
[220] Yang Zh-Yu., Burton D.J., J. Fluor. Chem., (2000), 102, 89-103.
[221] Wang Y., Parkin S.R., Giersehner J., Watson M.D., Org. Letters, (2008), 10, 3307-3310.
[222] Wood T.K., Waren W.E., Keay B.A., Parvez M., Angew. Chem., Int. Ed. Engl., (2009), 48, 4009-4012.
[223] Nitschke J. K., Tilley T.D., J. Am. Chem. Soc., (2001), 123, 10183-10190.
[224] Zahn A., Brotschi C., Leumann C.J., Chemistry – A Europ. J., (2005), 11, 2125-2129.
[225] Pat. WO 93467 (2006) (C.A., 2006, 145, 326323).
[226] Ishihara K., Hasegawa A., Yamamoto H., Angew. Chem., Int. Ed. Engl., (2001), 40, 4077-4079.
[227] Crouch D.J., Skabata P.J., Heeney M., Culloch I., Coles S.J., Hursthouse M.B., Chem. Commun., (2005), 1465-1467.
[228] Wang Y., Parkin S.R., Watson M.D., Org. Letters, (2008), 10, 4421-4424.
[229] Deck P.A., Lane M.J., Montgomery J.K., Slebodnick C., Frouczek F.R., Organometallics, (2000), 19, 1013-1024.
[230] Caster K.C., Keck Ch. G., Walls R.D., J. Org. Chem., (2001), 66, 2932-2936.
[231] Schlosser M., Guio L., Leroux F., J. Am. Chem. Soc., (2001), 123, 3822- 3823.
[232] Coe J.W., Wirtz M.C., Bashore C.G., Candler J., Org. Letters, (2004), 6, 1589- 1592.
[233] Pat. WO 128142 (2006) (C.A., 2007, 146, 27723).
[234] Pat. US 6362365 (2002) (C.A., 2000, 132, 308136).
[235] Tang G., Yang Y.G., Wen J.X., Mol. Cryst. Liquid Cryst. Science and Technology. Sect. A: Mol. Cryst. and Liquid Cryst., (2002), 373, 17-24.
[236] Pat WO 40131 (2006) (C.A., 2006, 144, 390561).
[237] Nuritomi M., Murofushi H., Nakashima N., Bull. Chem. Soc. Japan, (2004), 77, 2121-2128.
[238] Dutta T., Woody K.B., Watson M.D., J. Am. Chem. Soc., (2008), 130, 452- 453.
[239] Yan Sh-J., Huang Ch., Zeng X-H., Huang R., Lin J., Bioorg Med. Chem. Letters, (2010), 20, 48-51. 117
[240] Mallach E., Kuhn N., Maichle-Moessmer C., Steimann M., Stroebele M., Zeller K-P., Z. Naturforsch. B. Chemical Sciences, (2009), 64, 1176-1182.
[241] Pat. US 5856557 (1999) (C.A., 1998, 128, 88679).
[242]. Bella M., Kobbelgaard S., Jørgensen K.A., J. Am. Chem. Soc., (2005), 127, 3670-3671.
[243] Kobbelgaard S., Bella M., Jørgensen K.A., J. Org. Chem., (2006), 71, 4980- 4987.
[244] Steffen A., Sladek M.J., Braun T., Neumann B., Stammler H.G., Organometallics, (2005), 24, 4057-4064.
[245] Schaub T., Backes M., Radius U., J. Am. Chem. Soc., (2006), 128, 15964- 15965.
[246] Braun T., Perutz R.N., Sladek M.I., Chem. Commun., (2001), 2254-2255.
[247] Yoshikai N., Mashima H., Nakamura E., J. Am. Chem. Soc., (2005), 127, 17978-17979.
[248] Wang J-R., Manabe K., Org. Letters, (2009), 11, 741-744.
[249] Ishikawa S., Manabe K., Synthesis, (2008), 3180-3182; Manabe K., Ishikawa S., Synthesis, (2008), 2645-2649.
[250] Braun T., Isundu J, Steffen A., Neumann B., Stammler H-G., Dalton Trans., (2006), 5118-5123.
[251] Guo H., Kong F., Kanno K., He J., Nakajima K., Takahashi T., Organometallics, (2006), 25, 2045-2048.
[252] Korn T.J., Schade M.A., Cheemala M.N., Wirth S., Guevata S.A., Cahiez G., Synthesis, (2006), 3547-3574; Korn T.J., Schade M.A., Wirth S., Knochel P., Org. Letters, (2006), 8, 725-728.
[253] Edelbach B.L., Kraft B.M., Jones W.D., J. Am. Chem. Soc., (1999), 121, 10327-10331.
[254] Miller A.O., Krasnov V.I., Peters D., Platonov V.E., Miethchen R., Tetrahedron Letters, (2000), 41, 3817-3819.
[255] Buckley H.L., Sun A.D., Love J.A., Organometallics, (2009), 28, 6622-6624; Wang T., Love J.A., Organometallics, (2008), 27, 3290-3296.
[256] Blay G., Fernandez I., Monleon A., Pedro J-R., Vila C., Org. Letters, (2009), 11, 441-444. 118
[257] Musso D.L., Cochran F.R., Kelley J.L., McLean Ed.W., Selph J.L., Rigdon G.C., Orr G. Faye, Davis R.G., Cooper B.R., Styles V.L., Thompson J.B., Hall W.R., J. Med. Chem., (2003), 46, 399-408.
[258] Tagat J.R., McCombic S.W., Nazareno D.V., Boyll C.D., Kozlowski J.A., Chackalamannil S., Josien H., Wang Ya., Zhou G., J. Org. Chem., (2002), 67, 1171- 1177.
[259] Andrus M.B., Liu J., Tetrahedron Letters, (2006), 47, 5811-5814; Moran B.N., Anderson F.P., Cloonan S., Butler N.E., Kenny P.T.M., Deverg A., Verughese S., Draper S.M., Bioorg. Med. Chem., (2009), 17, 4510-4522.
[260] Cornella J., Lu P., Larrosa I., Org. Letters, (2009), 11, 5506-5509.
[261] Shang R., Fu Y., Wang Y., Xu Q., Yu H-Z., Liu L., Angew. Chem., Int. Ed. Engl., (2009), 48, 9350-9354.
[262] Wyatt P., Hudsen A., Charmant J., Orpen A.G., Phetmung H., Org. Biomol. Chem., (2006), 4, 2218-2232.
[263] Iovel I, Mertins K., Kirschel J., Zapf A., Beller M., Angew. Chem., Int. Ed. Engl., (2005), 44, 3913-3917.
[264] Li L., Chen H., Lin Y., Synth. Commun., (2007), 37, 985-991.
Recommended for publication by prof. V.E. Platonov
Fluorine Notes, 2014, 93, 1-2
