Received: February , 2014
Fluorine Notes, 2014, 93, 5-6
Perfluorocyclobutene and its dimers in Diels-Alder reaction with cyclopentadiene and furan. New data
A. Yu. Volkonskii,* E. M. Kagramanova, E. I. Mysov , N. D. Kagramanov
A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova
28, V-334, GSP-1, 119991 Moscow, Russia
e-mail: volkonskii@ineos.ac.ru
Abstract: Fluorinated norbornenes and 7-oxanorbornene, containing perfluorocyclobutane fragments, were synthesized by Diels-Alder reaction from perfluorocyclobutene or perfluorocyclobutene dimers and cyclopentadiene or furan.
Keywords: perfluorocyclobutene, perfluorocyclobutene dimers, cyclopentadiene, furan, Diels-Alder reaction.
Fluorinated polymers on the base of norbornene and its analogues were found to be promising materials for extreme UV lithography (see e.g. [1, 2]), and also for generation of gas-permeable [3] and ion-selective [4] membranes. In this work is given new data concerning synthesis of fluorinated norbornenes (1-3) and 7-oxanorbornene (4) (see scheme 1), which are prospective monomers for such polymers’ production.
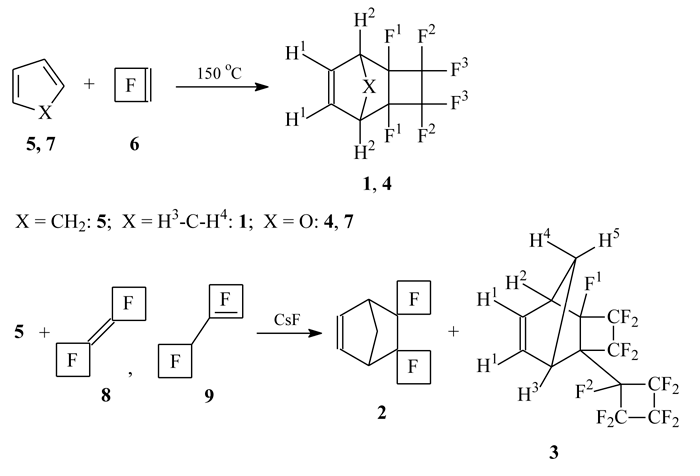
Scheme 1
It should be noted, that there are some single reports about present compounds in the literature. Thus, the hexafluorotricyclononene (1) obtaining was declared in the publication [5], and its hydroformylation was reported about in the article [6]. But nowhere was given neither detailed description of the compound (1) synthesis, nor its characteristics.
We obtained tricyclononene (1) as the mixture of endo- and exo-isomers (16:1) by prolonged heating of cyclopentadiene (CPD) (5) with the excess of perfluorocyclobutene (PFCB) (6) in autoclave without solvent, and the yield amounted to 34%. Similarly, by the heating of furan (7) with the excess of PFCB (6) was synthesized also 9-oxatricyclononene (4), isolated with the yield 23%. Low yield of products may be explained by necessity of end tricyclononene (1) deep purification from dicyclopentadiene (DCPD) impurity in the first case, and in the second case the reason was in the following [2+4]-cycloaddition reaction of compound (4) with furan (7) in this conditions (NMR and GC-MS data were not given).
The generation of 9-oxatricyclononene (4) by heating to 50-60 °C of ether solution of furan (7) and PFCB (6) equimolar mixture is described in the literature [7]. We didn’t succeed in replaying this experiment: the reaction almost failed by the temperature 50-100 °C and only about 150 °C the conversion of furan (7) became approximately 10% after 19 hours. Although the melting point and parameters of 1H and 19F NMR spectra of our compound obtained are differing from literary data [7], the totality of its spectral characteristics (NMR, Raman, MS, see Tables 1-3) allows us to assign it the [2+4]-cycloadduct structure (4).
The obtaining of dispironorbornene (2) by reaction of CPD (5) with PFCB dimer (8), or with the mixture of PFCB dimers (8/9) in the CsF presence [8, 9] is also described in the literature. So the authors claimed perfluorobicyclobutylidene (8) only to be able to undergo the Diels-Alder reaction, whereas the olefin (9) stayed inert, and only its isomerisation to dimer (8) occurred in presence of CsF [9]. But we found in the result of more thorough purification and examination of reaction products, that heating of CPD (5) with the mixture of PFCB dimers (8/9)* in the CsF presence, led to [2+4]-cycloadducts from both olefins: dispironorbornene (2) and 2-nonafluorocyclobutyl substituted tricyclononene (3), herewith the yield of dispironorbornene (2) beeing always higher at any ratio of starting dimers (8/9). Besides this it appeared that pure dispironorbornene (2) was solid, but not liquid as was reported in [9]**.
Polymerization of synthesized fluorinated norbornenes (1, 2) and also 7-oxanorbornene (4) was investigated in Philipps-University, Marburg, (FRG), by Prof. Heitz group [10].
Experimental
1H, and 19F (with CF3COOH as the external standard) NMR spectra were recorded in CDCl3 on Bruker WP-200SY spectrometer. Raman spectra were detected on Ramanor HG-2S spectrometer. Mass and GC-MS spectra were recorded on VG 7070E and Polaris/GCQ mass spectrometers (EI, 70 eV). Preparative GLC (PGLC) was performed at 150 В°C on QF1 on chromosorb column (l = 4 m, d =25 mm) or on FS1265 on chromosorb column (l = 3.6 m, d =25 mm).
CPD (5) and furan (9) were obtained by pyrolysis of DCPD and pyromucic acid, accordingly, and kept at -78 В°C. PFCB (6) [11] and PFCB dimers (8/9) with monohydrocycloalkane (10) impurity [12] were synthesized by known procedures, mixture ratio was evaluated according NMR spectra and GLC.
Synthesized compounds characteristics are represented in Tables 1-3.
2,3,3,4,4,5-Hexafluorotricyclo[4.2.1.02,5]nonene-7 (1)
The mixture of 5.93 g (89.7 mmol) CPD (5), 18.65 g (115.0 mmol) PFCB (6) and 0.2 g hydroquinone was heated at 150 °C for 72 h in autoclave. After cooling all volatile compounds were distilled off, the residue was filtered and autoclave was washed by small amount of ether. 11.94 g (58%) of cycloadduct (1) was obtained by combined filtrates distillating , purity ≥ 98%, DCPD impurity ~1.2% (NMR, GLC), b.p. 58-59 °C (20 Torr). PGLC (QF1) of distilled product gave 6.89 g (34%) of tricyclononene (1) as colorless liquid, representing the mixture of endo/exo-isomers (16:1), purity ≥99.9%, DCPD content ≤0.01% (NMR, GLC).
5-Norbornene-2-spiro-1’-(2’,2’,3’,3’,4’,4’-hexafluorocyclobutane)-3-spiro-1”-(2”,2”,3”,3”,4”,4”-hexafluorocyclobutane) (2) and 2-heptafluorocyclobutyl-3,3,4,4,5-pentafluortricyclo[4.2.1.02,5]nonene-7 (3)
The mixture of 1.78 g (27.0 mmol) CPD (5), 6.84 g PFCB dimers (8/9) with monohydrocycloalkane (10) [(8)/(9)/(10) = 1.0/0.51/0.15; 20.7 mmol calculating on C8F12] and 3.24 g (21.3 mmol) freshly calcined CsF were heated at 80 В°C for 16 h in periodically agitated sealed tube. After the reaction expired the supernatant liquid contained cycloadducts (2) and (3) in the ratio 4.4:1.0 (NMR, GLC).
Similarly, 3.19 g (48.3 mmol) CPD (5), 11.11 g PFCB dimers (8/9) with monohydrocycloalkane (10) [(8)/(9)/(10) = 1.0/0.82/0.33; 33.7 mmol calculating on C8F12] and 7.37 g (48.5 mmol) CsF, and also 0.45g (6.82 mmol) CPD (5), 1.49 g PFCB dimers (8/9) with monohydrocycloalkane (10) [(8)/(9)/(10) = 1.0/4.5/3.9; 4.39 mmol calculating on C8F12] and 1.18 g (7.76 mmol) CsF were heated for 6 h. The final solutions contained cycloadducts (2) Рё (3) in the ratio ~2:1 (NMR, GLC).
Reaction mixtures were filtered, the precipitate was washed with ether, combined filtrates were concentrated and distilled, the fraction with b.p. 61-66 °C (5 Torr) was collected. Distilled product PGLC (FS1265) gave 12.83 g (56%) of cycloadduct (2) as white solid, with purity ≥99%, DCPD content ≤0.01% (NMR, GLC), and also 2.04 g (9%) cycloadduct (3) as white fusible solid, with purity ≥99% (NMR, GLC).
2,3,3,4,4,5-Hexafluoro-9-oxatricyclo[4.2.1.02,5]nonene-7 (4)
The mixture of 9.6 g (0.141 mol) furan (7) and 44.7 g (0.276 mol) PFCB (6) was
heated at 150 В°C for 72 h in autoclave. After cooling all volatile compounds were distilled
off, the residue was solved in ether and filtered. Similarly , the ether solution of cycloadduct
(4) was obained from 4.4 g (0.065 mol) of furan (7) and 32.4 g (0.200 mol)
PFCB (6). The combined ether solutions were concentrated and the residue was distilled,
given 11.0 g (23%)
9-oxatricyclononene (4) as colorless liquid, hardened to white
crystalline substance with purity ≥99%.
Table 1. Boiling and melting points, Raman spectra, and elemental analysis data for the compounds obtained.
|
Compound |
B.p./В°C (p/Torr) [m.p./В°C] |
Raman, ν/cm–1 |
Found (%) Calculated(%) |
Molecular formula |
||
|
C |
H |
F |
||||
|
1 |
57.5 (20) |
1569.0 vs (C=C) |
47.3 47.4 |
2.50 2.65 |
50.2 50.0 |
C9H6F6 |
|
2 |
71.5-72 (7) [52-53]a |
- |
- |
- |
- |
- |
|
3 |
[31-33] |
1569.2 m (C=C) |
40.0 40.0 |
1.64 1.55 |
58.2 58.4 |
C13H6F12 |
|
4 |
68-69 (20) [50-51.5]b |
1568.5 vs (C=C) |
41.9 41.8 |
1.78 1.75 |
49.7 49.5 |
C8H4F6O |
a Colorless liquid [9]. b M.p. 59 В°C [7].
Table 2. NMR spectra of the compounds obtained.
|
Compound |
Оґ (J/Hz) |
|
|
1H |
19F |
|
|
1 endo-isomer |
1.71 (dtt, 1H, H(4), 2JH(4),H(3) = 11.6, 4JH(4),F(1) = 6.8, 5JH(4),F(2 or 3) = 2.1); 1.89 (br.d, 1H, H(3), 2JH(3),H(4) = 11.6); H(3),H(4) – AB-system; 3.38 (s, 2H, H(2)); 6.54 (s, 2H, H(1)) |
-112.5 (br.dt, 2F, F(1), 4JF(1),H(4) ≈ |
|
1 exo- isomer |
2.24 (br.d, 1H, H(3), 2JH(3),H(4) = 9.8); 2.39 (br.dm, 1H, H(4), 2JH(4),H(3) = 9.8); H(3),H(4) – AB-system; 3.22 (br.m, 2H, H(2)); 6.18 (br.m, 2H, H(1)) |
-110.2 m, 2F, F(1)); -49.7and -47.8 (both dm, both 2F, F(2) Рё F(3), AB-system, |
|
3 |
1.72 (br.dm, 1H, H(5), 2JH(5),H(4) = 11.6); 2.16 (br.d, 1H, H(4), 2JH(4),H(5) = 11.6); H(4),H(5) – AB-system; 3.47 и 3.56 (both br.s, both 1H, H(2) и H(3)); 6.63 (br.s, 2H, H(1)) |
-101.5 (br.d.d, 1F, F(1), Jd = 51.5, Jd = 28.6);
-94.8 (br.s, 1F, F(2)); |
|
4 |
5.28 (br.s, 2H, H(2)); 6.84 (br.s, 2H, H(1))Р° |
-114.5 (m, 2F, F(1)); -52.5 (m, 4F, |
Р° For the 1H Рё 19F NMR spectra, cf. Ref. [7].
Table 3. Mass spectra of the compounds obtained.
|
Com-pound |
m/z (Irel. (%)) |
|
1 endo- isomer |
228 [M]+ (8.7); 209 [M – F]+ (0.6); 189 [C9H5F4 and/or C6H3F6]+ (1.7); 169 [C6H2F5]+ (2.6); 163 [C7H3F4]+ (3.0); 145 [C7H4F3]+ (3.5); 128 [M – C2F4]+ (8.7); 127 |
|
1 exo- isomer |
228 [M]+ (15.7); 209 [M – F]+ (1.1); 189 [C9H5F4 and/or C6H3F6]+ (2.4); 177 |
|
2 |
390 [M]+ (2.3); 351 [C13H5F10 and/or C10H3F12]+ (3.2); 331 [C13H4F9 and/or C10H2F11]+ (5.6); 311 [C13H3F8 and/or C10HF10]+ (6.6); 290 [M – C2F4]+ (22.8); 289 [M – C2HF4]+ (25.4); 275 [C7HF10]+ (16.4); 269 [C11H4F7]+ (23.2); 239 [M – C3HF6]+ (28.0); 231 [C8H2F7]+ (22.7); 221 [M – C3F7]+ (26.6); 219 [C10H4F5]+ (41.4); 201 [C10H5F4]+ (49.4); 200 [C10H4F4]+ (33.9); 190 [C9H6F4]+ (30.9); 189 [C9H5F4]+ (36.4); 170 [C6H3F5]+ (43.0); 169 [C6H2F5]+ (50.9); 151 [C6H3F4]+ (40.1); 66 [C5H6]+ (100); 65 [C5H5]+ (39.4)a |
|
3 |
390 [M]+ (6.1); 371 [M – F]+ (2.5); 351 [C13H5F10 and/or C10H3F12]+ (4.6); 331 [C13H4F9 and/or C10H2F11]+ (5.8); 311 [C13H3F8 and/or C10HF10]+ (6.1); 301 [C12H5F8]+ (6.8); 290 [M – C2F4]+ (17.2); 289 [M – C2HF4]+ (27.6); 269 [C11H4F7]+ (20.2); 239 [M – C3HF6]+ (25.9); 219 [C10H4F5]+ (39.7); 201 [C10H5F4]+ (44.0); 200 [C10H4F4]+ (32.7); 189 [C6H3F6]+ (25.6); 170 [C6H3F5]+ (46.0); 169 [C6H2F5]+ (46.6); 151 [C6H3F4]+ (31.6); 115 [C6H5F2]+ (37.9); 66 [C5H6]+ (100); 65 [C5H5]+ (44.8) |
|
4 |
191 [C8H3F4O and/or C5HF6O]+ (0.2); 181 [C7H2F5]+ (0.6); 163 [C4HF6]+ (3.4); 132 [C3HF5]+ (4.3); 114 [C3H2F4]+ (3.0); 101 [C2HF4]+ (4.8); 93 [C3F3]+ (3.6); 75 [C3HF2]+ (4.8); 68 [C4H4O]+ (100); 57 [C3H2F]+ (3.4); 51 [CHF2]+ (3.4); 40 [C3H4]+ (6.1); 39 [C3H3]+ (8.7); 31 [CF]+ (5.5); 29 [CHO]+ (6.9) |
a For the mass spectrum, cf. Ref. [9].
*The mixture of PFCB dimers (8/9) used in this work contained some amount of hydrofluorination
product –
1-hydro-1-nonafluorcyclobutyl-2,2,3,3,4,4-hexafluorocyclobutane (10),
which quantatively underwent dehydrofluorination in presence of CsF to fluoroolefins (8/9) in situ
**Parameters of 1H and 19F NMR spectra of dispironorbornene (2) obtained here virtually coincide with literary data [9].
References
- C. Chang, D. Barnes, L. D. Seger, L. F. Rhodes, R. P. Lattimer, G. M. Benedikt, J. Photopolym. Sci. Technol., 2012, 25, 161-169.
- T. Yamashita, M. Morita, Y. Tanaka, J. J. Santillan, T. Itani, J. Photopolym. Sci. Technol., 2011, 24, 165-172.
- J. Vargas, A. A. Santiago, M. A. Tlenkopatchev, M. Lopez-Gonzalez, E. Riande, J. Membr. Sci., 2010, 361, 78-88.
- A. A. Santiago, J. Vargas, J. Cruz-Gomez, M. A. Tlenkopatchev, R. Gavino, M. Lopez-Gonzalez, E. Riande, Polymer, 2011, 52, 4208-4220.
- V. A. Al’bekov, V. N. Mironov, A. F. Benda, P. F. Potashnikov, G. A. Sokol’sky, Abstr. of V National Organofluorine Conference (May 20-22 1986), Moscow, 1986, p. 3.
- I. I. Kerov, V. A. Yashkir, G. A. Korneeva, I. I. Krylov, E. V. Slivinskii, A. V. Ignatenko, Petroleum Chemistry, 1998, 38, 156-163 (Engl. Transl.).
- V. A. Al’bekov, A. F. Benda, A. F. Gontar’, G. A. Sokol’sky, I. L. Knunyants, Bull. Acad. Sci. USSR, Div. Chem. Sci., 1988, Vol. 37, No. 4 (Engl. Transl.).
- R. D. Chambers, J. R. Kirk, G. Taylor, R. L. Powell, J. Fluor. Chem., 1983, 22, 393-395.
- A. E. Bayliff, M. R. Bryce, R. D. Chambers, J. R. Kirk, G. Taylor, J. Chem. Soc., Perkin Trans. 1, 1985, 1191-1193.
- T. Haselwander, Dissertation, Marburg, 1996.
- Syntheses of Organofluorine Compounds, Eds. I. L. Knunyants, G. G. Yakobson, Khimiya, Moscow, 1973, p. 46.
- R. D. Chambers, G. Taylor, R. L. Powell, J. Chem. Soc., Perkin Trans. 1, 1980, 426-428.
Recommended for publication by Prof. N.D. Chkanikov
Fluorine Notes, 2014, 93, 5-6
