Received: December , 2013
Fluorine Notes, 2014, 93, 3-4
Novel approach to the synthesis of Оі-carbolines and 5-azaindoles using 3,3,3-trifluoro-1-nitropropene
Fedorovskiy O.Yu.,a Gusev D.V.,a Chkanikov N.D.,a Ivanov A.V.,b Scherbakova V.S.b
aA.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova
28, V-334, GSP-1, 119991 Moscow, Russia
e-mail: offskii@rambler.ru
bIrkutsk Institute of Chemistry named after A.E.Favorskiy SO RAS,ul.Favorskogo, 1, 664033, Irkutsk, Russia,Р•-mail: ivanov@irioch.irk.ru
Abstract: Reported is the preparation of some novel fluoroderivatives of Оі-carbolines and 5-azaindoles using 3,3,3-trifluoro-1-nitropropene.
Keywords: Fluorinated Оі-carbolines, fluorinated 5-azaindoles, 3,3,3-trifluoro-1-nitropropene, 4,5,6,7-tetrahydroindole, Pictet-Spengler cyclization.
Earlier we have offered a convenient method for the synthesis of condensed 3-trifluorometilpiridines using 3,3,3-trifluoro-1-nitropropene (2). Thus, ОІ-carbolines were synthesized starting with indole and alkene (2). The key intermediates were tryptamine derivatives that resulted from the reduction of the products of indole C3-alkylation with alkene (2) [1]. Recently we have shown that derivatives of 4,5,6,7-tetrahydroindole (THI) (1a), that is a substituted pyrrole, now easily available [2], are regioselectively and quantitatively oxyalkylated at C2 carbon atoms in their pyrrole rings under the action of polyfluorinated carbonyl compounds [3]. The result allowed us hope that C2-alkylation of THI with alkenes (2) opens the way to iso-tryptamine derivatives and further to fluorinated Оі-carbolines, those being of great interest as promising drug preparations [4, 5, 6]. The present study makes it evident that indeed the said synthesis scheme results in the production of saturated derivatives of dihydro-, tetrahydro-, and octahydro-Оі-carbolines, precoursors of aromatic Оі-carbolines (see scheme 2). The latter are productable through the dehydrogenation of saturated Оі-carbolines and withdrawal of protecting groups by well-known methods [7]. The same transformation scheme was applied in this our study for the synthesis of fluorinated 5-azaindoles (see scheme 3), those being of undoubted interest in the search of new medical formulations [8, 9]. Following [6] we made use of the general numbering of the produced substances (see scheme 2).
Discussion of results.
The interaction of THI with 3,3,3-trifluoro-1-nitropropene (2) in absolute ether solution results in an unstable product that decomposes within few hours even at decreased temperature (between minus 70 and 0В°C) in argon environment. The replacement of THI with N-vinyl-THI in the reaction with alkene (2) does not increase the stability of nitro-adduct (3).
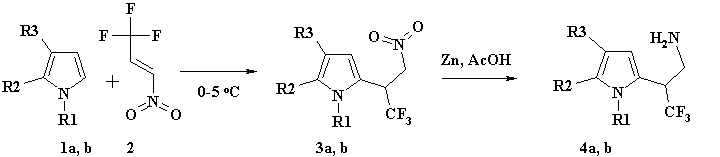
Scheme 1. (a: R1=Bnz, R2-R3= -(CH2)4-; b: R1= -Me, R2=R3= H)
The reaction product (3a) proved to be stable in the case of THI with nitrogen atom protected by benzyl. Therefore, N-Bnz-THI (1a) smoothly reacted with 1,1,1-trifluoro-3-nitropropene (2) at the second position of pyrrol ring by in absolute ether at 0В°C in argon environment with quantitative yield. Thus produced nitro-derivative (3a) was reduced to N-Bnz-iso-tryptamine (4a) with activated zinc dust in acetic acid solution (see scheme 1). Further, N-Bnz-iso-tryptamine (4a) was introduced into the cyclization with СЂ-phenyl-3-trifluoromethyl-benzaldehyde at the conditions of Pictet-Spengler reaction [10] (see scheme 2).
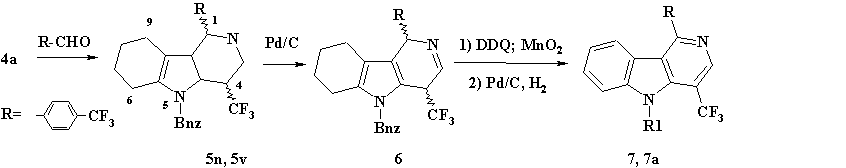
Scheme 2. ((7) R1= Bnz; (7a) R1= H).
The reaction resulted in 2 diastereomers (5n) and (5v) at ratio 6/4 correspondingly, that were separated by chromatography on silicagel. The total yield of (5n) and (5v) was 48%. It should be mentioned that during cyclization in acetic acid solution in the presence of catalytic amounts of СЂ-toluenesulfoacid only one Оі-carboline diastereomer is formed (5v) with yield of 50%.
Under the conditions of catalytic dehydration, the reactivity of (5n) and (5v) diastereomers differs, that is applicable to the difference in spatial structure of their pyridine rings. Thus, boiling of diastereomer (5n) in benzene solution in the presence of 10% Pd/C resulted in its dehydration with a double bond formation in its pyridine ring that was substance (6). At the same conditions substance (5v) was dehydration-resistant. Long-time boiling of (5n, 5v, 6) in 1,3,5-trimethylbenzene in the presence of DDQ resulted in low yield of aromatic substance (7) (see scheme 2). The use of activated manganese dioxide for dehydration of (5n) or (5v) in 1,3,5-trimethylbenzene solution resulted in 67% yield of substance (7). The protecting benzyl group was withdrawn by boiling (7) in ethanol solution in the presence of 10% Pd/C in hydrogen environment; it resulted in the formation of (7a) with yield of 65%.
5-Azaindoles [11, 12] prepared in this study are of undoubtedly interest as well in the search of novel medical formulations. The transformation scheme applied in our synthesis of fluorinated 5-azaindoles was similar to that of Оі-carbolines manufacture when starting with N-methylpyrrol (1b) and alkene (2) (see scheme 1).
Similar to N-Bnz-THI or N-methylpyrrol the reaction at 0В°C was regioselective by C2 carbon in pyrrole ring, resulting in nitrofluoroderivative (3b), with nearly quantitative yield, however, in [13] at the same conditions the researchers did not observe regioselectivity. NitrocompoundРµ (3b) was reduced to 3,3,3-Trifluoro-2-(1-methyl-1H-pyrrol-2-yl)-propylamine (4b) by activated zinc in aqueous solution of acetic acid with good yield (83%). The substance was introduced into the cyclization with aldehydes under mild conditions (~50 В°C) according to Pictet-Spengler reaction (see scheme 3) resulting in the formation of derivatives of 4,5,6,7-tetrahydro-pyrrolpyridines (8, 9). Those were prepared as a blend of diastereomers. After purification and separation by preparative chromatography on silicagel the main diastereomers were isolated with yields 65% and 55% for compounds (8) and (9), correspondingly.
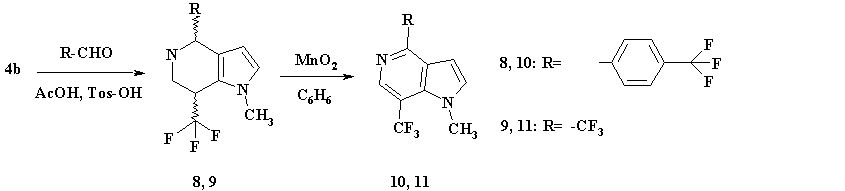
Scheme 3.
Dehydrogenation of (8) and (9) was conducted in the presence of activated manganese dioxide in boiling benzene solution and resulted in the formation of 5-azaindole derivatives (10, 11) with high yields (see scheme 3).
Therefore, we propose a scheme for the synthesis of annulated pyridines using 3,3,3-trifluoro-1-nitropropene (2) allows the manufacture of fluorinated derivatives of saturated and aromatic Оі-carbolines and 5-azaindoles.
Experiment:
Both purity and structure of the prepared substances were confirmed by thin-layer chromatography, with Kieselgel plates 60 F254, NMR-spectroscopy Avance 300, for 1H, 19F, and by chromato-mass-spectrometry with Finnigan Polaris Q/Trace GC ultra, ionic trap, EU 70eV. Column: RTX-5ms, phase type: 5% phenylpolysilphenylene-siloxane.
1-Benzyl-2-(2,2,2-trifluoro-1-nitromethyl-ethyl)-4,5,6,7-tetrahydro-1H-indole (3a).
To 100 ml of ether solution containing 7.1g (0.0336mole) of N-Bnz-THI (1a) we added 4.74g (0.0336mole) of 1-nitro-3,3,3-trifluoropropene (2) at 0-5 В°C. The reaction was TLC-controlled, eluent: chloroform/ethylacetate at ratio 95/5. After completing the process (about 3 hours) the ether solution was evaporated on a rotor evaporator under decreased pressure. Compound (3a) was produced in amount of 11.8g with virtually quantitative yield, and was used in further transformations without additional purification. Light yellow oil, Rf= 0.75.
Spectrum NMR-1H, dmso-d6: 1.6ppm, 4H, m, 5, 6 -CH2-; 2.2-2.4ppm, 4H, m, 4, 7 -CH2-; 4.6-4.7ppm, 1H, m, *CH-CF3; 4.9-5.3ppm, 4H, Ph-CH2-, N-CH2-; 6.11ppm, 1H, s, C3H; 6.9-7.3ppm, 5H, m, Bnz.
Spectrum NMR-19F, dmso-d6: 9.8ppm, d, j=10Hz, -CF3 .
Mass-spectrum: m/z 352 [M]+ (19).
2-(1-Benzyl-4,5,6,7-tetrahydro-1H-indole-2-РёР»)-3,3,3-trifluoro-propylamine (4a).
11g (0.031mole) of nitrocompound (3a) was dissolved in 70ml of 50% aqueous acetic acid, and 10g of activated zinc was added by small portions during 15 minutes. The reaction was conducted in temperature range between 40 to 50В°C under continuous stirring. The reaction was TLC-controlled till complete disappear of original substance (3a). After that the reaction mass was cooled down to room temperature and diluted with ether (300 ml). Ethereal extract was then separated from aqueous solution of zinc acetate and alkalified with saturated ~70% aqueous potash solution. As soon as СЂH of aqueous salt solution under ether coat equaled 10, the ether solution containing N-Bnz-iso-tryptamine (4a) was stripped off, and the raw product was purified in a silicagel-filled column; eluent: dichloromethane/methanol = 95/5. It resulted in 7g of substance (4a) with yield 70%. Light yellow oil, Rf= 0.5.
Spectrum NMR-1H, dmso-d6: 1.5-1.8ppm, 4H, m, 5, 6-CH2-; 2.2-2.5ppm, 4H, m, 4, 7-CH2-; 2.7-3.1ppm, 2H, m, N-CH2-; 3.2-3.4ppm, 3H, s broadened, -NH2; 3.5-3.6ppm, 1H, m, *CH; 5.1ppm, 2H, d, J=7Hz, Bnz-CH2-; 5.9ppm, 1H, s, C3H, 6.9-7.3ppm, 5H, m, Bnz.
Spectrum NMR-19F, dmso-d6: 9.6ppm, d, j=10Hz, -CF3.
Mass-spectrum: m/z 322 [M+] (23).
5-Benzyl-4-trifluoromethyl-1-(4-trifluoromethyl-phenyl)-2,3,4,5,6,7,8,9-octahydro-1H-pyrido[4,3-b]indole (5).
1.43g (0.0088 mole) of 4-trifluoro-methylbenzaldehyde and 0.5ml of concentrated muriatic acid were added to 25 ml of ethanol solution containing 2.656g (0.0082 mole) of N-Bnz-iso-tryptamine (4a). The reaction mass was being boiled for 3 hours under stirring with a reflux condenser. After the process completion (TLC-controlled) surplus muriatic acid was neutralized with 2g of potash, the reaction mass was filtered and stripped off, and organics that contained a blend of two diastereomers of 1,2,3,4,6,7,8,9-octahydro-Оі-carboline (5n, 5v) was purified with the help of chromatography on silicagel, with diastereomer separation. Eluent: dichloromethane/petroleum ether, at ratio 9/1. Substance (5n) was isolated in amount of 1.2g, and substance (5v) in amount of 0.7g. Total yield of Оі-carboline (5), taking into account both diastereomers, was 1.9g, 48%.
Substance (5n): TLC: eluent chloroform/ethylacetate at ratio 97/3, Rf= 0.3.
Spectrum NMR-1H, dmso-d6: 1.3-1.6ppm, 4H, m, 7, 8 -CH2-; 2.1-2.3ppm, 4H, m, 6, 9 –CH2-; 2.9-3.1ppm, 2H, m, C3H2; 3.4ppm, 1H, d, J=11Hz, C1H; 3.6ppm, 1H, m, C4H; 4.95ppm, 1H, s, broadened, NH; 5.05ppm, 2H, d, J=7Hz, Bnz-CH2-; 6.9-7.3ppm, 5H, m, Bnz, 7.5-7.7ppm, 4H, 2d, J=7Hz, p-Ph.
Spectrum NMR-19F, dmso-d6: 13.1ppm, 3F, d, j=10Hz, -CF3; 17.6ppm, 3F, s, Ph-CF3.
Mass-spectrum: m/z 478 [M]+ (100); 407 [M-CF3]+ (91), 380 [M-Bnz]+.
SubstanceРµ (5v): TLC: eluent chloroform/ethylacetate at ratio 97/3, Rf= 0.7.
Spectrum NMR-1H, dmso-d6: 1.4-1.6ppm, 4H, m, 7, 8 -CH2-; 2.1-2.3ppm, 4H, m, 6, 9 -CH2-; 2.7ppm, 2H, m, C3H2; 3.1ppm, 1H, d, J=12Hz, C1H; 3.45ppm, 1H, m, C4H; 4.95ppm, 1H, s, broadened, NH; 5.1ppm, 2H, d, J=10Hz, Bnz-CH2-; 6.8, 7.6ppm, 4H, d, J=10Hz, p-Ph; 7.2-7.4ppm, 5H, m, Bnz.
Spectrum NMR-19F, dmso-d6: 13.7ppm, 3F, d, j=10Hz, -CF3; 17.2ppm, 3F, s, Ph-CF3.
Mass-spectrum: m/z 478 [M]+ (65); 387 [M-Bnz]+ (71); 380 [M-Bnz-7H]+ (81).
5-Benzyl-4-trifluoromethyl-1-(4-trifluoromethyl-phenyl)-4,5,6,7,8,9-гексaгидро-1H-pyrido[4,3-b]indole (6).
30mg of 10% Pd/C was added to 80mg (0.000167mole) of substance (5n) dissolved in 10ml of benzene, and the reaction mass was being stirred and boiled with a reflux condenser for 3 hours. The process was TLC-controlled, eluent: chloroform/ethylacetate at ratio 95/5. The solvent was then stripped off at a rotor evaporator under decreased pressure, and the residuum was chromatographically purified at silicagel, eluent: chloroform/ethylacetate at ratio 95/5. It resulted in the production of 50mg of substance (6) with yield 63%.
Spectrum NMR-1H, dmso-d6: 1.5-1.7ppm, m, 4H, 7,8 –CH2-; 2.0-2.2ppm, m, 6, 9 -CH2-; 3.7ppm, 1H, dd, J=10Hz, C3H; 4.05ppm, 1H, m, C4H; 4.45ppm, 1H, d, J=20Hz, C1H; 5.21ppm, 2H, s, Bnz-CH2-; 6.95-7.3ppm, 5H, m, Bnz; 7.6-7.8ppm, 4H, 2d, J=10Hz, p-Ph.
Spectrum NMR-19F, dmso-d6: 13.1ppm, 3F, d, j=10Hz, -CF3; 17.3ppm, 3F, s, Ph-CF3.
Mass-spectrum: m/z 476 [M]+ (6), 474 [M-2H]+ (14), 470 [M-6H]+ (18).
5-Benzyl-4-trifluoromethyl-1-(4-trifluoromethyl-phenyl)-5H-pyrido[4,3-b]indole (7).
Procedure (a). 60mg of DDQ were added to 10ml of 1,3,5-trimethylbenzene solution that contained 20mg (4.2*10-5 mole) of (5n) and was being boiled during 72 hours. After disappearance of original carboline the solution was stripped off, and the residuum was purified on silicagel, eluent: dichloromethane/petroleum ether (49/70) at ratio 8/2. ~5mg of (7) was isolated, yield 25%.
Procedure (b). The mixture of 75mg (0.000157mole) (5n) and 75mg (0.000157mole) (5v) was being boiled in the presence of 300mg activated manganese dioxide in 15ml of 1,3,5-trimethylbenzene during 3 hours under continuous stirring till complete disappearance of original (5), the reaction was TLC-controlled. Further it followed procedure (a). 100mg of substance (7) was isolated, yield 67%.
Spectrum NMR-1H, dmso-d6: 5.8ppm, 2H, s, Bnz-CH2-; 6.9-7.6ppm, 9H, m, Bnz-, H6, H7, H8, H9; 7.8-8.00ppm, 4H, 2d, J=10Hz, p-Ph-; 8.95ppm, 1H, s, H3.
Spectrum NMR-19F, dmso-d6: 15.1ppm, 3F, s, -CF3; 22.6ppm, 3F, s, -CF3.
Mass-spectrum: m/z 470 [M]+ (36), 91 [Bnz]+ (100).
4-trifluoromethyl-1-(4-trifluoromethyl-phenyl)-5H-pyrido[4,3-b]indole (7a).
100mg of 10% Pd/C and 100mg of 36% HCl was added to 10ml of ethanol solution that contained 150mg (3.2*10-4 mole) of substance (7), and switched on a hydrogen box with pressure ~0.5 atm. The reaction blend was being intensively stirred and heated to 50В°C during 24 hours. The reaction was TLC-controlled till complete disappearance of original (7). Then the reaction blend was filtered on zeolite, and the solution was stripped off at a rotor evaporator, 30ml of water and 3g of sodium bicarbonate was added. The blend was extracted with ether (30ml x 3 times), the ethereal extracts was pooled, dried with waterless sodium sulfate, and stripped off at decreased pressure. The product was purified on 50g of silicagel, eluent: dichloromethane/petroleum ether at ratio 8/2, Rf= 0.6. 80mg of substance (7a) was isolated, with yield 65%.
Spectrum NMR-1H, dmso-d6: 7.1-7.7ppm, 4H, m, C6H-C9H; 7.8-8.0ppm, 4H, 2d, J=10Hz, p-Ph; 8.37ppm, 1H, s broadened, NH, 8.67ppm, 1H, s, C3H.
Spectrum NMR-19F, dmso-d6: 16.27ppm, 3F, s, -CF3; 17.94ppm, 3F, s, -CF3.
Mass-spectrum: m/z 380 [M]+ (69), 379 [M-H]+ (100), 359 [M-HF]+ (27).
3,3,3-Trifluoro-2-(1-methyl-1H-pyrrol-2-yl)-propylamine (4b).
3g of activated zinc was during 5 minutes added by small portions to 25ml of 50% aqueous solution of acetic acid that contained 3.45g (0.0155mole) of (3b). The reaction blend was then heated to 40-50°C at continuous stirring during 3 hours. Both isolation and purification of substance (4b) were similar to those for substance (4a). Получено 2.4г (4b), yield 83%. Light yellow oil, Rf=0.2, eluent chloroform/ethylacetate at ratio 9/1.
Spectrum NMR-1H, dmso-d6: 2.3ppm, 2H, s, broadened, -NH2; 3.1-3.4ppm, 2H, m, -CH2-; 3.55-3.55ppm, 1H, m, *CH; 3.61ppm, 3H, s, N-Me; 6.13-6.17ppm, 2H, m, CH, 6.63ppm, 1H, s, CH.
Spectrum NMR-19F, dmso-d6: 8.2ppm, d, J=8Hz, -CF3.
Mass-spectrum: m/z 192 [M]+ (37).
1-Methyl-7-trifluoromethyl-4-(4-trifluoromethyl-phenyl)-4,5,6,7-tetrahydro-1H-pyrrolРѕ[3,2-c]pyridine (8).
0.35Рі (0.002mole) of СЂ-phenyl-3-trifluoromethylbenzaldehyde and catalytic amount of СЂ-toluene-sulfoacid (10mg) were added to 10ml of acetic acid solution that contained 0.384g (0.002mole) of (4b). The reaction mass was being heated at 50В°C for two hours. After that the reaction mass was stripped off in a rotor evaporator under decreased pressure and moved into 25ml of water that contained 3g of potash. Its aqueous layer was extracted with ether, tree times each with 20ml, the ethereal extracts were put together and dried with unhydrous sodium sulfate. By further purification on silicagel, eluent chloroform/ethylacetate at ratio 9/1, the chief diastereomer (8) was isolated in amount of 0.45g with yield 65%. Slow crystallizing oil, Rf=0.3.
Spectrum NMR-1H, dmso-d6: 2.7-2.9ppm, 1H, m, C6H; 3.0-3.15ppm, 1H, m, C6H; 3.4-3.5ppm, 1H, d, J=15Hz, C4H; 3.54ppm, 3H, s, N-Me; 3.7-3.8ppm, 1H, m, C7H; 4.92ppm, 1H, s, NH; 5.46ppm, 1H, d, J=3Hz, C3H; 6.65ppm, 1H, d, J=3Hz, C2H; 7.5-7.7ppm, 4H, 2d, J=8Hz.
Spectrum NMR-19F, dmso-d6: 13.8ppm, 3F, d, J=13Hz, C7-CF3; 17.6ppm, 3F, s, p-Ph-CF3.
Mass-spectrum: m/z 348 [M]+ (27).
1-Methyl-4,7-РІРёСЃ-trifluoromethyl-4,5,6,7-tetrahydro-1H-pyrrolРѕ[3,2-c]pyridine (9).
The preparation procedure was similar to that of (8). 0.3g of substance (9) was isolated, with yield 55%. Slow crystallizing oil, Rf=0.7, eluent chloroform/ethylacetate at ratio 9/1.
Spectrum NMR-1H, dmso-d6: 2.9-3.1ppm, 2H, m, C6H2; 3.3-3.4ppm, 1H, m, C4H; 3.54ppm, 3H, s, N-Me; 3.75-3.85ppm, 1H, m, C7H; 4.45-4.55ppm, 1H, m, broadened, NH; 5.95ppm, 1H, s, broadened, C3H; 6.8ppm, 1H, d, J=3Hz, C2H.
Spectrum NMR-19F, dmso-d6: -64.5ppm, 3F, d, J=23Hz, -CF3; -75.5ppm, 3F, d, J=23Hz , -CF3.
Mass-spectrum: m/z 272 [M]+ (34).
1-Methyl-7-trifluoromethyl-4-(4-trifluoromethyl-phenyl)-1H-pyrrolРѕ[3,2-c]pyridine (10).
50mg of activated manganese dioxide were added to 10ml of benzene solution that contained 30mg (8.62*10-5mole) of substance (8). The reaction mass under intensive stirring with a reflux condenser was being boiled for two hours. The reaction was TLC-controlled. Then benzene was stripped off in a rotor evaporator, and the residuum was purified using chromatography on silicagel, eluent: chloroform/ethylacetate at ratio 9/1. After purification 25mg of (10) was isolated with yield 85%. Slow crystallizing oil, Rf=0.9, eluent chloroform/ethylacetate at ratio 9/1.
Spectrum NMR-1H, dmso-d6: 3.97ppm, 3H, s, N-Me; 6.96ppm, 1H, s, broadened, C3H; 7.73ppm, 1H, s, broadened, C2H; 7.9-8.3ppm, 4H, 2d, J=5Hz, p-Ph; 8.71ppm, 1H, s, C6H.
Spectrum NMR-19F, dmso-d6: 17ppm, 3F, s, -CF3; 25.5ppm, 3F, s, -CF3.
Mass-spectrum: m/z 344 [M]+ (85).
1-Methyl-4,7-РІРёСЃ-trifluoromethyl-1H-pyrrolРѕ[3,2-c]pyridine (11).
The preparation procedure was similar to that of (10). 42mg of substance (11) was isolated, with yield 87%. Slow crystallizing oil, Rf=0.9, eluent chloroform/ethylacetate at ratio 9/1.
Spectrum NMR-1H, dmso-d6: 4.02ppm, 3H, s, N-Me; 6.9ppm, 1H, s, C3H; 7.9ppm, 1H, d, J=3Hz, C2H; 8.75ppm, 1H, s, C6H.
Spectrum NMR-19F, dmso-d6: -53.5ppm, 3F, s, -CF3; -64.5ppm, 3F, s, -CF3.
Mass-spectrum: m/z 268 [M]+ (71).
References
2. (a) R. J. Tedeschi, Acetylene, in Encyclopedia of Physical Science and Technology, 3rd Edition, ed. R. A. Meyers, Acad. Press, Inc.: San Diego, 2001, vol. 1, p. 55-89; (b) Z. Wang Comprehensive Organic Name Reactions and Reagents. Part 3. Wiley, London, 2009, p. 2793-2796; (c) Name Reactions in Heterocyclic Chemistry. II, ed.: J. J. Li, Wiley-VCH, Hoboken, New Jersey, 2011, p. 72-82; (d) B. A. Trofimov, A. I. Mihaleva, E. YU. SHmidt, L. N. Sobenina, Himiya pirrola, Novye stranicy, Nauka, Novosibirsk, 2012, 382 s; (e) Trofimov B.A., Mihaleva A.I., SHmidt E.YU. i dr., Pat. RF N 2297410, Byull. Izobr. 2007. N 11.
3. A.L. Sigan, D. V. Gusev, N.D. Chkanikov, E.Yu. Shmidt, A.V. Ivanov, A.I. Mihaleva., Hydroxyalkylation of 4,5,6,7-tetrahydroindole with polyfluorocarbonyl compounds as a route to 2-substituted indoles. Tetrahedron Letters, 52, (2011), 5025–5028.
4. A.N. Kost, M.A. Yurovskaya, F.A. Trofimov, Chem. Heterocyclic. Comp., 9, 267, (1973).
5. R.S. Alekseev, A.V. Kurkin, M.A. Yurovskaya.γ-karboliny i ih gidrirovannye proizvodnye. 1. Aromaticheskieγ-karboliny: metody sinteza, himicheskie i biologicheskie svojstva (obzor).HGS, 2009, N8, s.1123-1166.
6. R.S. Alekseev, A.V. Kurkin, M.A. Yurovskaya. γ-karboliny i ih gidrirovannye proizvodnye.2. Gidrirovannye proizvodnyeγ-karbolinov: metody sinteza (obzor). HGS, 2010, N7, s.963-1018.(а)
7. A.V. Butin, A.S. Pilipenko, A.A. Milich, A.V. Fin'ko, Chem. Heterocycl. Comp., 45, 613, (2009); (b) Protective Groups in Organic Synthesis, Third Edition.Theodora W. Greene, Peter G.M. Wuts Copyright. 1999, John Wiley & Sons, Inc.
8. Shumaila, Abdullah M. A.; Kusurkar, Radhika S.; Puranik, Vedavati G.; Tetrahedron; vol. 67; nb. 5; (2011); p. 936- 942.
9. Yokoyama, Naota; Arai, Takayoshi; Chemical Communications (Cambridge, United Kingdom); nb. 22; (2009); p. 3285 – 3287.
10. Vacuro K.V., Mishchenko G.L., Imennye reakcii v organicheskoj himii., Moskva, «Himiya», 528 str, 1976g.
11. R. S. Alekseev, A. V. Kurkin, M. A. Yurovskaya, Aza-γ-karboliny i ih benzannelirovannye proizvodnye: metody sinteza, himicheskie i biologicheskie svojstva., HGS, 2011, N2, s.1765-1801.
12. Yahontov L.N., "Himiya geterociklicheskih soedinenij", 1982, N 9, s. 1155-67.Evers Rainer; Klenz Oliver; Miethchen Ralf; Michalik Manfred., Organofluorine compounds and fluorinating agents Part 16: Monoalkylations and cycloadditions withtrans-3,3,3-trifluoro-1 nitropropene Journal of Fluorine Chemistry, 1997 , vol. 81, # 2 p. 205 – 210.
13. Evers Rainer; Klenz Oliver; Miethchen Ralf; Michalik Manfred., Organofluorine compounds and fluorinating agents Part 16: Monoalkylations and cycloadditions with trans-3,3,3-trifluoro-1 nitropropene Journal of Fluorine Chemistry, 1997 , vol. 81, # 2 p. 205 – 210.
Recommended for publication by Prof. N.D. Chkanikov
Fluorine Notes, 2014, 93, 3-4
