Received: December, 2013
Fluorine Notes, 2013, 91, 9-10
Obtaining of pentafluorophenol by oxidation of Trimethyl(pentafluorophenyl)silane
V. E. Boykoab, A.A. Tyutyunovab, V. L. Donab, S. M. Igoumnovab
aA.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, V-334, GSP-1, 119991 Moscow, Russia
b ZAO NPO” P&M-Invest”, 119334, Moscow, Leninsky avenue 47
e-mail: boykii@mail.ru
Abstract: A new method for obtaining of pentafluorophenol by oxidation of trimethyl(pentafluorophenyl)silane is developed.
Keywords: pentafluorophenol, hexafluorobenzene, (pentafluorophenyl)trimethylsilane
Pentafluorophenol and its derivatives are widely used for making polymers, with unique properties [1], synthesis of aminoacids, peptides and nucleosides, which are the intermediates in synthesis of antitumor agents, HIV inhibitors [2-4], as components of metallocene catalysis systems for olefin polymerization [5].
All till the present known methods for obtaining of pentafluorophenol are based on the reaction of nucleophilic substitution of fluorine atom in hexafluorobenzene with potassium hydroxide [6-9] or alcoholates [10].
Currently hexafluorobenzene is not available in commercial quantities, as production of hexachlorobenzene, from which it was obtained, is prohibited. That’s why there appeared a necessity in development of conceptually new approach to the synthesis of pentafluorophenol on the base of available raw materials.
A method for obtaining of pentafluorophenol through reaction of splitting of borates with hydrogen dioxide in alkaline condition is described in the literature. Its main disadvantage is the necessity to use corresponding Grignard reagent for obtaining of (pentafluorophenyl)trimethylborate, which significantly complicates the scale-up of this method [11, 12].
Previously we developed a simple and effective method for obtaining of (pentafluorophenyl)trimethylsilane from pentafluorobenzoic acid or pentafluorobromobenzene [13]. That’s why we found it interesting to study a possibility of oxidation of (pentafluorophenyl)trimethylsilane in pentafluorophenol.
Varying the nature of oxidizing agents and conditions of reaction, we found out that pentafluorophenol is obtained with an acceptable yield 65 % only by usage of tert-butylperoxybenzoate as an oxidizing agent and carrying out the reaction in the presence of KF and CuCl in DMF. By usage of other oxidizing agents the formation of pentafluorbenzene and decafluorobiphenyl in different proportions was observed in Table 1.
Table 1. Effect of oxidizing agent
|
# |
Peroxide |
Yield % |
||
|
PhfH |
PhfOH |
PhfPhf |
||
|
1 |
PhC(O)OOtBu |
22 |
65 |
13 |
|
2 |
t BuOOH |
89 |
0 |
11 |
|
3 |
t BuOOtBu |
91 |
0 |
9 |
|
4 |
H2O2 |
100 |
0 |
0 |
|
5 |
CO(NH2)2H2O2 |
84 |
0 |
16 |
|
6 |
BaO2 |
73 |
0 |
27 |
|
7 |
MnO2 |
49 |
3 |
48 |
|
8 |
CrO3 |
72 |
0 |
28 |
|
9 |
Na2S2O8 |
100 |
0 |
0 |
It is necessary to point out that there is no oxidation of (pentafluorophenyl)trimethylsilane by tert-butylperoxybenzoate in the absence of potassium fluoride or CuCl. In this case the main product of reaction is pentafluorobenzene.
Scheme 1
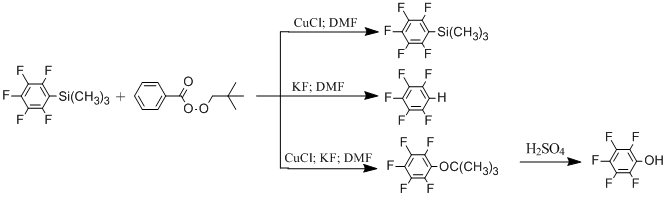
Another salts of monovalent cooper unlike salts of divalent cooper also catalyze the reaction of oxidation.
Table. 2 The effect of catalyst
|
# Exp. |
Cat. |
Yield% |
||
|
PhfOH |
PhfH |
PhfPhf |
||
|
1 |
- |
0 |
100 |
0 |
|
2 |
CuCl |
65 |
22 |
13 |
|
3 |
CuBr |
52 |
30 |
18 |
|
4 |
CuI |
55 |
27 |
18 |
|
5 |
CuSO4 |
0 |
100 |
0 |
|
6 |
CH3O2SOH |
0 |
0 |
0 |
|
7 |
H2SO4 |
0 |
0 |
0 |
By studying the effect of solvent on the flow of this reaction, it was found out that the best solvent for this reaction is DMF.
Table 3. The effect of solvent
|
# |
Solv. |
Yield % |
||
|
PhfOH |
PhfH |
PhfPhf |
||
|
1 |
DMF |
65 |
22 |
13 |
|
2 |
N-methylpyrrolidone |
58 |
22 |
20 |
|
3 |
Tetramethylurea |
0 |
100 |
0 |
|
4 |
Sulfolane |
31 |
35 |
34 |
|
5 |
Acetonitrile |
30 |
51 |
19 |
|
6 |
DMAA |
0 |
100 |
0 |
Influence of temperature on carrying out the process was also studied. It is appeared that the best yield of pentafluorophenol was obtained by carrying out the process at heat of 20 °C , and it amounts 65 %. Tert-butylperoxybenzoate doesn’t enter into reaction by 0 °C, and the following warming of reaction mixture to room temperature leads to uncontrolled exothermic reaction up to 110 °C. If the reaction is carried out by 50 – 80 °C, the output of target product reduces to 20 %.
As a result we developed a new method for obtaining of pentafluorophenol from readily available (pentafluorophenyl)trimethylsilane. We also optimized conditions for carrying out the process in commercial scales.
Experimental
NMR 1H, 19F spectra are taken on spectrometer “Bruker AVANCE-300” by 300 and 282 MHz correspondingly, ESTD is CDCl3. Chemical shifts for 1H spectra are indicated relative to solvent’s residual signal (δ 7.25, 4.79) and are given per million relative to TMS. Chemical shifts of 19F spectra are given per million relative to CFCl3.
Put 500 ml of absolute DMFA in 2 L flask and while intensively stirring add 58 g (1 mol) of potassium fluoride and 9.9 g (0.1 mol) of cooper chloride. Then from drop funnel add 240 g (1 mol) of (pentafluorophenyl)trimethylsilane to the reaction mixture, which must be cooled to 10 °C. Stir reaction mixture during 15 – 20 minutes and warm to 20 °C and then dose from drop funnel 194 g (1 mol) of tertbutylperoxybenzoate with such a speed, so that the temperature of reaction mixture don’t exceed 20 °C. Then the reaction mixture is dissolved with equal volume of 1 N HCl. The lower layer is separated and rerun. 119 g of pentafluorophenol are obtained. The boiling point is 143 °C. The melting point is 34-36 °C. NMR 1H δ: 10.5 (s, 1H,); NNR 19F δ: -165,1 (s, 2F 2,6), -168,3 (s, 2F 3,5), -174,7(s, 1F 4). Found (%): C, 39,07; H, 0,61; F, 51,69. C6HF5O. Calculated (%): C, 39,15; H, 0,55; F, 51,61
References
- B. Boutevin, A. Rousseau, D. Bosc; J. Polym. Sci. A, Polym.Chem., 1992, 30(7), 1279-1286;
- M. S. Ashwood, B. C. Bishop, I. F. Cottrell, K. M. Emerson, D. Hands, J. G. Ho, J. E. Lynch, Y. J. Shi, R. D. Wilson; USA Patent # 7262169, 2007;
- R. P. Beckett, M. Whittaker, A. Miller, F. M. Martin; WO 9616931 1996;
- T. Sammakia, T. B. Hurley; J. Org. Chem., 2000, 65(4), 979;
- F. A.R Kaul, G. T Puchta, H. Schneider, M. Grosche, D. M. W. A Herrmann; J. Organomet. Chem., 2001, 621, 184;
- L. A.Wall, W. J.Pummer, J. E.Fearn, J. M.Antonucci; J. Res. NBS, 1963, 67A, 481-497;
- I.K. Bildin, D.F. Mukhametsin S.I. Konovalov, A.A. Zakharov, M.M. Zinnurov; RF Patent # 2343142 C2, 2009;
- J. M. Birchall, R. N. Haszeldine; J. Chem. Soc., 1959, 13-17;
- W. J. Pummer, L. A. Wall; Science, 1958, 127, 643;
- E.J. Forbes, R.D. Richardson, M. Stacey, J.C. Tatlow; J. Chem. Soc., 1959, 2019–2021;
- T.Korenaga, F.Kobayashi, K.Nomura, S.Nagao, T.Sakai; J. Fluorine Chem., 2007, 128, 1153–1157;
- I.Ikuyo, M.Hitoshi; Japanese Patent Application JP 82548(A), 2005;
- V. E. Boyko, A. A. Tyutyunov, V. L. Don, S. M. Igumnov; Preparative method for obtaining of (polyfluoroaril)trimethylsilanes //Fluorine notes, 2013, 6 (91).
Recommended for publication by Prof. S. Igoumnov
Fluorine Notes, 2013, 91, 9-10
