Received: November, 2013
Fluorine Notes, 2013, 91, 7-8
Preparative method for obtaining of (polyfluoroaryl)trimethylsilanes
V. E. Boykoab, A.A. Tyutyunovab, V. L. Donab, S. M. Igoumnovab
aA.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, V-334, GSP-1, 119991 Moscow, Russia
b ZAO NPO "P&M-Invest", 119334, Moscow, Leninsky avenue 47
e-mail: boykii@mail.ru
Abstract: There were developed two preparative methods for obtaining of (polyfluoroaryl)trimethylsilanes from industrially readily available fluorinated aromatic acids.
Keywords: (polyfluoroaryl)trimethylsilanes, (pentafluorophenyl)trimethylsilane, (tetrafluorophenyl)bis(trimethyl)disilanes, (tetrafluorophenyl)trimethylsilanes.
(Polyfluoroaryl)trimethylsilanes are versatile reagents for induction of fluoroaromatic fragments into organic molecules. Compounds containing these fragments are used in electronics and strongly demanded in industry for producing diodes [1], LCDs [2] etc; they are also used in printing [3]. But methods for obtaining of these compounds are unsuitable for large-scale production. Thereby the purpose of our study was to develop a new, technologically simple way to obtain (polyfluoroaryl)trimethylsilanes from starting materials, available in commercial quantities.
Synthetic significance of organofluorinesilanes led to the emergence of a number of works devoted to the method development for their obtaining. Thus, the synthesis of silane 3a has been successfully done by electrochemical silylation of pentafluorobenzene, using soluble anodes of zinc or aluminum [10]. Fluoroaryltrimethylsilanes are formed with good yield through reaction of Grignard reagents [4, 5] or lithium analogues [6, 7] with trimethylchlorosilane. The alternative reaction is reaction of polyfluoroarylhalides with trimethylchlorosilane in the presence of trisdialkilamidaphosphines [8]. Also (pentafluorophenyl)trimethylsilane can be obtained by reaction between pentafluorobenzoylchloride and hexamethyldisilane over palladium complex catalysis with PdCl2(PhCN)2 in the presence of triethylphosphite [9].
All above listed methods of obtaining silane 3a have significant disadvantages. They can be hardly used in production as they require the use of highly flammable solvents, organometallic compounds, which are stable only in an inert atmosphere [4-7], secondly - highly toxic, carcinogenic substances [8], and at last - significant quantities of expensive catalysts [9], as well as specialized complex equipment [10].
We suggest to use easily accessible fluorinated aromatic acid as starting synthons. Their potassium salts, interacting with trimethylchlorosilane in a polar aprotic solvent such as dimethylformamide, dimethylacetamide, N-methylpyrrolidone or sulfolane, at a temperature of 95-130В°C form appropriate (polyfluoroaryl)trimethylsilanes 3a-h with a good yield.
We choose the reaction of obtaining of (pentafluorophenyl)trimethylsilane 3a as a typical one. Within it we studied the effect of solvent and temperature.
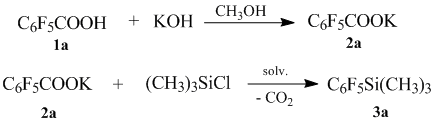
The obtained data are shown in Table 1. There we can see that the best results were obtained by carrying out the process in DMF.
Table 1 Influence of the solvent.
|
# |
solvent |
TВ°C |
Yield % |
|
1 |
DMF |
95 |
86 |
|
2 |
DMAA |
110 |
79 |
|
3 |
N-methylpyrrolidone |
105 |
81 |
|
4 |
Sulfolane |
130 |
81 |
|
5 |
Acetonitrile |
80 |
0 |
Model reaction was extended to other fluorine-containing mono-and dicarboxylic acids.
Target products can be obtained from the salts of the fluorinated aromatic diacids. And they can contain one or two trimethylsilyl groups according to the number taken for the reaction of trimethylchlorosilane. When using two-fold molar excess against the trimethylchlorosilane dipotassium salt fluoroaromatic diacid, the (tetrafluorophenyl)bis(trimethyl)disilanes are obtained with a yield of 73-80%.
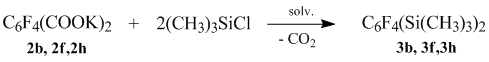
At equimolar ratio tetrafluorophenylsilane, polyfluorophenyl(trimethyl)silanes, identical to tetrafluorophenylcarbone are produced acids with the yield of 81-83%.
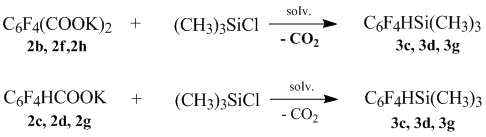
The results are shown in Table 2.
Table 2. Obtaining of fluoroaryl(trimethyl)silanes from fluoroaromatic acids potassium salts.
|
# |
RfCOOH (1a-h) |
RfCOOK (2a-h) |
RfCOOK/ClSi(CH3)3 (mol) |
Yield , |
|
1 |
1a |
2a |
1 / 1 |
(3a)В 86 |
|
2 |
1b |
2b |
1 / 2 |
(3b)В 80 |
|
3 |
1c |
2c |
1 / 1 |
(3c)В 55 |
|
4 |
1b |
2b |
1 / 1 |
(3d)В 83 |
|
5 |
1d |
2d |
1 / 1 |
(3d)В 82 |
|
6 |
1e |
2e |
1 / 1 |
(3e)В 73 |
|
7 |
1f |
2f |
1 / 1 |
(3c)В 38 |
|
8 |
1g |
2g |
1 / 1 |
(3g)В 81 |
|
9 |
1h |
2h |
1 / 1 |
(3g)В 81 |
|
10 |
1h |
2h |
1 / 2 |
(3h)В 76 |
|
11 |
1f |
2f |
1 / 2 |
(3f)В 32 |
Two mechanisms of this process flow are possible.
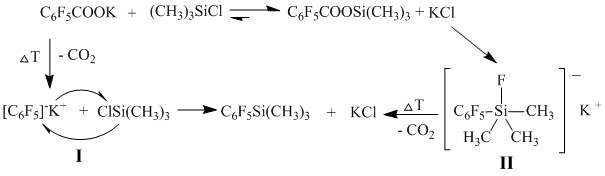
For clarifying it, we developed an alternative way of production of (polyfluorophenil)trimethylsilanes from trimethylsilyl esters of fluorinated aromatic acids in the presence of a nucleophile.
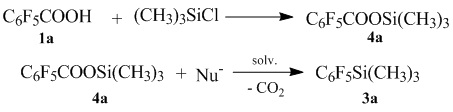
When the reaction is conducted without a catalyst (in the absence of nucleophile), decarboxylation does not occur even at higher temperatures.
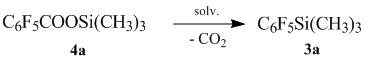
We tested various solvents within the model for obtaining (pentafluorophenyl)trimethylsilane, so that we can determine the optimum process conditions. The results are shown in Table 3.
Table 3. Effect of solvent.
|
# Experim. |
Solvent |
Yield, RfSi(CH3)3, % |
|
1 |
DMF |
(3a)В 89 |
|
2 |
DMAA |
(3a)В 80 |
|
3 |
Sulfolane |
(3a)В 95 |
|
4 |
N-methylpyrrolidone |
(3a)В 88 |
Good results were obtained in all tested solvents, but the best results were achieved in sulfolane where the effect of the nucleophile and its amount, required for the process have been studied.
Table 4. Effect of the nucleophile and its amount.
|
# Experim. |
Nu- |
RfCOOSi(CH3)3В / Nu- |
Yield RfSi(CH3)3, % |
|
1 |
NaF |
1/1 |
(3a)В 20% |
|
2 |
KF |
1/1 |
(3a)В 95% |
|
3 |
KF |
1/0.1 |
(3a)В 92% |
|
4 |
KF |
1/0.01 |
(3a)В 89% |
|
5 |
CsF |
1/0.1 |
(3a)В 93% |
|
6 |
KCl |
1/0.01 |
(3a)В 91% |
All nucleophiles except sodium fluoride showed excellent results. Also from the obtained data it can be concluded that 1% of nucleophile is enough for the complete reaction transmission. In this way it was found out that the formation of silane 3a occurs through a mechanism II via decarboxylation silylether 4a. Attempting to scale-up in the presence of fluoride ion, as the nucleophile, after decarboxylation of trimethylsilylpentafluorobenzoate leads to defluoromethylsililation with the formation of pentafluorophenyl polymer, which is not observed in the case of chlorine ion as a nucleophile.
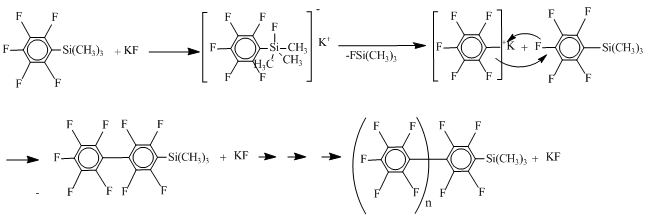
The results obtained within the study of model reaction have been extended to other fluorine-containing mono-and dicarboxylic acids. Calculations were conducted using DMF as a solvent and potassium chloride as nucleophile. The obtained results are shown in Table 5.
Table 5. Obtaining (fluoroaryl)trimethylsilanes from the fluorine-containing aromatic acids through formation of the corresponding trimethylsilyl esters.
|
# |
RfCOOH (1a-h) |
RfCOOH : ClSi(CH3)3 (mole) |
RfCOOSi(CH3)3 (4a-h) |
Yield, RfSi(CH3)3 (3a-h), % |
|
1 |
1a |
1:3 |
4a |
(3a) 84 |
|
2 |
1b |
1:6 |
4b |
(3b) 83 |
|
3 |
1c |
1:3 |
4c |
(3c) 25 |
|
4 |
1b |
1:1 |
4d |
(3d) 83 |
|
5 |
1g |
1:3 |
4g |
(3g) 86 |
|
6 |
1d |
1:3 |
4d |
(3d) 83 |
|
7 |
1e |
1:3 |
4e |
(3e) 75 |
|
8 |
1h |
1:1 |
4g |
(3g) 79 |
|
9 |
1f |
1:1 |
4c |
(3c) 26 |
|
10 |
1h |
1:6 |
4h |
(3h) 81 |
|
11 |
1f |
1:6 |
4f |
(3f) 28 |
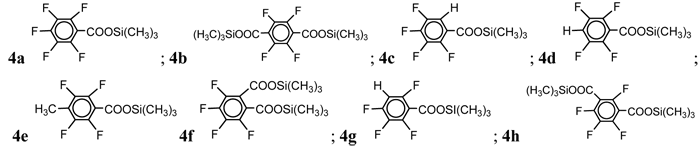
Experimental
General method for the synthesis of fluorinated arylsilanes from corresponding potassium salts of fluorinated carboxylic acids.
Potassium hydroxide 23.7 g (0.42 mol) is dissolved in 150 ml of methanol. Add a solution (0.42 mol) of fluorine-benzoic acid 1 into 200 mL of methanol while stirring at room temperature. After mixing for 30 minutes filter and airdry the precipitate. Get the potassium salt of benzoic acid fluoride 2.
While stirring add the resulting potassium salt of fluorine-benzoic acid 2 (0.36 mol) into 100 ml of solvent, then add 47.7 g (0.44 mol) of trimethylchlorosilane. After the reaction mixture was heated to 70 В° C, held for 1 hour and then the temperature adjusted prior to gassing and held at this temperature until it is finished, the solution was cooled, poured into water and the lower layer was separated, dried over magnesium sulfate, filtered and distilled.
General method for the synthesis of fluorinated arylsilanes out of corresponding silyl esters of carboxylic acids
While stirring add fluorine-containing carboxylic acid 1 (1.25 mol) to 150 g (1.38 mol) of trimethylchlorosilane, heat it till boiling, control the rate of gas. Then distill off the excess of trimethylchlorosilane and distill the product in vacuum. Add 100 mL of a solvent and 0.3 g (0.004 mol) of potassium chloride to the resulting (trimethyl)silyl ether of fluorinated benzoic acid 4 (0.08 mol). Heat the mixture up till gas starts isolating and carry on until the process is finished. Afterwards cool the mixture to a room temperature then separate the lower layer, dry it over magnesium sulfate, filter and distill.
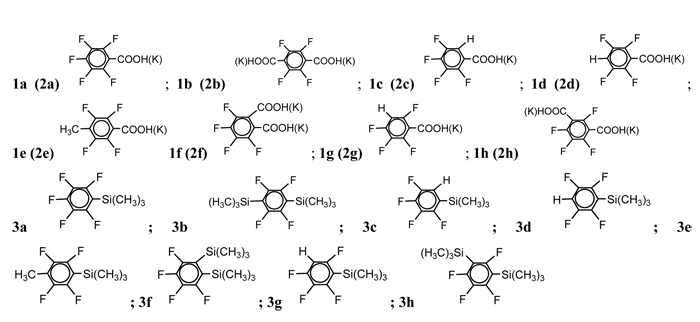
The spectral silanes data is given in the literature: 3Р° [11], 3b [13], 3c [13], 3d [13,14,15], 3e 3f [11], 3g [12], 3h [12].
Trimethyl(2,3,5,6-tetrafluoro-4-methylphenyl)silane, (3e).
The b.p. is 198 В°C. Found (%): C, 50,79; H, 5,06; F, 32,27. C10H12F4Si. Calculated (%): C, 50,85; H, 5,08; F, 32,20. NMR 1H Оґ: 0.55 (s, 9H, Si(CH3)3), 2.15 (s, 3H, CH3); NMR 19F Оґ: -128,5 (s, 2F 2,6), -140,2 (s, 2F 3,5)
References
- V.A. Montes, G. Li, R. Pohl, J. Shinar, P. Anzenbacher Jr; Adv.Mater., 2004, 16, 2001;
- M. Matsui, K. Shirai, N. Tanaka, K. Funabiki, H. Muramatsu, K. Shibata, Y. Ohgomori, Y. Ohgomori, J. Mater. Chem., 1999, v.9, p. 2755
- S.L. Malhotra, US PatentРђ 1998, 5744273;
- M. Fild, O. Glemser, G. Christoph, Angewandte Chemie, 1964 76, p. 953;
- C. Edmonson, A.E. Jukes, H. Gilman, Journal of Organometallic Chemistry, 1970, 25, 273-276;
- C. Tamborski, E.J. Soloski, Journal of Organometallic Chemistry, 1969, 17, 185-192;
- A.J. Oliver, W.A.G. Graham, Journal of Organoelemental Chem., 1969, 19, 17-27;
- V.V. Bardin, L.S. Pressman, L.N. Rogoza, G.G. Furin, J. Gen. Chem. USSR, 1992, 62, 2342-2349;
- A.. Stepanov, B.I. Martynov, A.P. Tomilov, Electrochemistry, 2000, 36, 210-218;
- Kashiwabara, M. Tanaka, Organometallics, 2006, 25, 4648-4652;
- X. Chen, V. Kumar, D.M. Lemal, S. Ramanathan, D. Sang; J. Org.Chem., 2012, 77, 2, 966-970;
- Kuroda, Ishikawa; Nippon Kagaku Zasshi; 1970, , 91, 489- 494;
- Tamborski, Soloski; J. Org.Chem., 1969, 17, 185-188;
- Bardin; Russian Chemical Bulletin., 1997, 46, 4, 780-785;
- M. Fujita, M. Obayashi, T. Hiyama; Tetrahedron, 1988, 44, 13, 4135-4146;
Recommended for publication by Prof. S. Igoumnov
Fluorine Notes, 2013, 91, 7-8
