Received: November, 2013
Fluorine Notes, 2013, 91, 3-4
Synthesis and Biological Activity of Permethrin Analogs Containing the 4-methoxy-2,3,5,6-tetrafluorobenzene Group in the Alcoholic Moiety
O.I.В Lukashov1, N.A.В Sokolova1, S.N.В Golosov1, N.E.В Kuzmina2, P.V.В Kazakov1, N.S.В Mirzabekova1
1) State Research Institute of Organic Chemistry and Technology, Shosse Entuziastov
23, Moscow, 111024 Russia,
e-mail: dir@gosniiokht.ru
2)State Budgetary Institution “Scientific Center for Expertise of Medical Application Products” of the Ministry of Health of the Russian Federation, Petrovskiy bul’var 8, Moscow,127051, Russia.
Abstract: A series of permethrin analogs were synthesized containing the 4-methoxy-2,3,5,6-tetrafluorobenzyl group as the alcoholic component and various substituents: CH3, C2H5, C3H7, C6H5CH2, (CH2)n (nВ =В 4, 5) in position 2 of the cyclopropane ring. The insecticidal activity of the novel compounds against typhoid flies, rice weevils, and black bean aphid was estimated. The examined compounds were shown to possess weak insecticidal activity.
Keywords: permethrin, 4-methoxy-2,3,5,6-tetrafluorobenzyl, insecticidal activity.
Pyrethroids are analogues of natural pyrethrins – cyclopropane carboxylic acid esters possessing insecticidal activity. Research in the field of pyrethroids, including the synthesis of novel pyrethroid compounds [1–4], is in progress. The conventional trend in the synthesis of new pyrethroids consists in varying the ester group. In recent years literature data appeared which are evidence of high insecticidal activity of chrysanthemic and permethric acid analogues containing the 4-methoxy-2,3,5,6-tetrafluorobenzyl group in the alcoholic moiety [5–9].
It should be noted that another approach to preparing biologically active pyrethroid structures, i.e. by the substitution of the gem-dimethyl group in position 2 of the cyclopropane ring for various functional substituents, is also reported [9–14]. As we previously showed, analogues of ethyl- and 3-phenoxybenzyl esters of permethric acid having alkyl and phenylalkyl substituents in position 2 of the cyclopropane ring possessed a weak insecticidal activity, exhibiting at the same time pronounced juvenile hormone activity not typical of pyrethroids [15, 16]. It was assumed that the introduction of the fluorobenzyl group into the alcoholic moiety of pyrethroid compounds would allow one to increase significantly their insecticidal activity.
The main object of the present work was to obtain derivatives of 4-methoxy-2,3,5,6-tetrafluorobenzyl permetric acid ester containing both various alkyl- and benzylalkyl substituents, and spiro derivatives in position 2 of the cyclopropane ring, and to explore their biological activity.
4-Methoxy-2,3,5,6-tetraftorbenzilcyclopropane carboxylates (II) were prepared by interacting 4-methoxy-2,3,5,6-tetrafluorobenzyl bromide with the corresponding sodium salt of 2,2-disubstituted permethric acid, , (I) [15], heating the reaction mixture for 8 to 10 hours at 100В°C according to the following scheme:
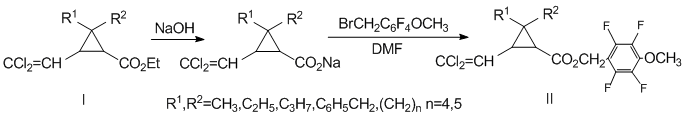
Individual substances were isolated by column chromatography. The resulting compounds (IIa-f) were pale yellow oily substances, insoluble in water and readily soluble in common organic solvents. The structure of the novel compounds was confirmed by IR, NMR spectroscopy (1H, 13C, 1H-1H COSY (Correlation Spectroscopy) [16], 1H-13C HSQC (Heteronuclear Single Quantum Correlation) [17], GC-mass spectrometry, and elemental analysis.
The spatial configuration of 4-methoxy-2,3,5,6-tetrafluorobenzyl-cyclopropane carboxylates of 2,2-disubstituted permethric acid was determined using two-dimensional ROESY spectra [18]. The data obtained are presented in the experimental part.
Studies of the biological activity [20] proved that the obtained compounds exhibited a weak insecticidal activity against model insects, and in a concentration of 0.1% showed close insecticidal activity values. Synthesized for comparison 4-methoxy-2,3,5,6-tetrafluorobenzylic derivatives of chrysanthemic and permethric acids (IIa and IIb) exhibited a high insecticidal activity against typhoid flies exceeding that of tetramethrin and permethrin, taken as reference, by the factor of 5 and 9, respectively.
From the data on biological activity it can be concluded that the introduction of the fluorobenzyl group into the alcohol moiety of permethric acid analogues having different substituents in position 2 of the cyclopropane ring does not increase the insecticidal activity. Apparently, the presence of the dimethyl group in position 2 of the cyclopropane ring is decisive for a pyrethroid to manifest insecticidal activity. However, in modern literature there is lack of reliable data on the role of the dimethyl group; it remains unclear whether it has purely structural functions or participates in the formation of the receptor site.
EXPERIMENTAL
NMR spectra were recorded on a Bruker Avance-300 spectrometer at 300 MHz at room temperature with the use of CDCl3 as solvent and TMS as internal reference. The IR-spectra were taken on a Perkin-Elmer 1720 spectrometer in CCl4 or in thin film. The chromatomass spectra (electron impact) were recorded on a Fisons Trio 1000 system GC-MS with a quadrupole mass analyzer. HPLC analysis was performed on a Millikhrom AO-2 liquid chromatograph equipped with a spectrophotometric detector and reversed-phase column. The column chromatography was performed on Silica gel 60 (0,040–0,063 mm; Merck) using a Kontes glass column with an inner diameter of 48 mm and length of 300 mm, eluents: hexane–benzene (1:1), benzene–ether (9:1). The check of the purity and individuality of the compounds and monitoring the progress of the reactions were performed on of Silica gel 60 F254 plates (Merck).
4-Methoxy-2,3,5,6-tetrafluorobenzyl bromide and DMF were commercial products. Ethanol was absolutized according to the procedure described in [21].
General Procedure. A solution of 0.1 mmol of 2,2-disubstituted permethric acid ethyl ester (I) and 0,11 mmol of sodium hydroxide in 200 ml of ethanol was boiled for 3h. The alcohol was then distilled off under a reduced pressure, and the solid residue was dissolved in 150 ml of DMF. To the solution 0.11 mmol of 4-methoxy-2,3,5,6-tetrafluorobenzyl bromide was added and the mixture was heated at 100°C for 8h. DMF was then removed in vacuo on a rotary evaporator; the residue was diluted with 150 ml of benzene and washed with water (3X100 ml). Benzene was distilled off in vacuo on the rotary evaporator. The residue – pale yellow oily product – was chromatographed on a preparative column, eluent: hexane–benzene. The content of the main substance was determined by HPLC.
2,2-Dimethyl-3-(2-methyl-1-propenyl)cyclopropane acid, 4-ethoxy-2,3,5,6-tetrafluorobenzyl ester (IIa). Yield 83% (61% after chromatographic separation); the product contained 96% of the main substance. IR spectrum, ν, cm–1: 1735 (C=O ester), 1620 (C=C). 1H NMR spectrum, δ, ppm: (1R*,3R*) isomer: 1.16 s (3H, CH3), 1.21 s (3H, CH3), 1.61 d (1H, CH), 1.63 s (3H, CH3), 1.66 s (3H, CH3), 1.87 t (1H, CH), 4.05 s (3H, OCH3), 5.12 d (2H, OCH2), 5.33 d (1H, CH=); (1R*,3S*) isomer: 1.09 s (3H, CH3), 1.23 s (3H, CH3), 1.35 d (1H, CH), 1.66 s (3H, CH3), 1.70 s (3H, CH3), 2.04 t (1H, CH), 4.05 s (3H, OCH3), 4.85 d (1H, CH =), 5.12 d (2H, OCH2). 13C spectrum, δ, ppm: (1R*,3R*) isomer: 14.50, 17.97, 26.55, 28.40, 28.87, 30.72, 32.43, 52.63, 61.73 m (4J 4.2 Hz), 107.83 m (2J 17.4 Hz), 117.77, 134.91, 138.85 t (2J4.2 Hz), 139.60 d (1J 248.6 Hz), 142.30 d (1J 248.6 Hz), 144.23 d (1J 248.6 Hz), 147.42 d (1J 248.6 Hz), 170.14, (1R*,3S*) isomer: 18.14, 20.08, 21.81, 25.21, 28.86, 33.01, 34.25, 53.05, 61.78 t (4J 4.2 Hz), 107.83 t (2J 17.4 Hz), 120.79, 135.61, 138.85 t (2J 4.2 Hz), 141.0, d (1J 248.6 Hz), 145.90 d (1J 248.6 Hz), 170.01. Mass spectrum: m/z 360 [M]+. Found, %: C 59.75; H 5.39; F 21.49. C18H20F4O3. Calculated, %: C 60.00; H 5.59; F 21.09. M 360.35.
3-(2,2-Dichloroethenyl)-2,2-dimethylcyclopropane acid, 4-methoxy-2,3,5,6-tetrafluorobenzyl ester (IIb). Yield 81% (57% after column separation), the product contained 96% of the main substance. IR spectrum, ν, cm–1: 1735 (C=O ester.), 1620 (C=C). 1H NMR spectrum, δ, ppm: (1R*,3R*) isomer: 1.19 s (3H, CH3), 1,30 s (3H, CH3), 1.85 d (1H, CH), 2.05 q (1H, CH), 4.09 s (3H, OCH3), 5.20 s (2H, OCH2), 6.26 d (1H, CH=); (1R*,3S*) isomer: 1.23 s (3H, CH3), 1.36 s (3H, CH3), 1.67 d (1H, CH), 2.33 dd (1H, CH), 4.13 s (3H, OCH3), 5.25 s (2H, OCH2), 5.65 d (1H, CH=). 13C spectrum, δ, ppm: (1R*,3R*) isomer: 15.37, 22.60, 27.71, 30.68, 31.36, 53.84, 62.07 t (4J 4.2 Hz), 107.41 t (2J 17.4 . Hz), 120.85, 124.61, 139.01 t (2J 4.2 Hz), 140.81 d (1J 248.6 Hz), 145.94 d (1J 248.6 Hz), 170.71; (1R*,3S*) isomer: 19.99, 22.46, 29.35, 33.20, 34.36, 53.66, 62.08 t (4J 4.2 Hz), 107.41 t (2J 17.5 Hz), 122.27, 126.82, 139.01 t (2J 4.8 Hz), 140.81 d (1J 246.8 Hz), 145.77 d (1J 248.6 Hz), 170.31. Mass spectrum: m/z: 401 [M]+. Found, %: C 47.78; H 3.32; F 19.11; Cl 17.82. C16H14F4Cl2O3. Calculated, %: C 47.90; H 3.52; F 18.94; Cl 17.67. M 401.19.
3-(2,2-Dichlorethenyl)-2-ethyl-2-methylcyclopropane acid, 4-methoxy-2,3,5,6-tetrafluorobenzyl ester (IIc). Yield: 76% (52% after column separation), the product contained 98% of the main substance. IR spectrum, ν, cm–1: 1735 (C=O ester), 1620 (C=C). 1H NMR spectrum, δ, ppm: (1R*,2R*,3S*) isomer: 0.89 t, (3H, CH3), 1.23 s (3H, CH3), 1.36 m (2H, CH2), 1.67 d (1H, CH), 2.33 dd (1H, CH), 4.13 s (3H, OCH3), 5.25 s (2H, OCH2), 5.65 d (1H, CH=); (1R*,2R*,3R*) isomer: 1.01 s (3H, CH3), 1.19 s (3H, CH3), 1.30 m (2H, CH2), 1.85 d (1H, CH), 2.05 q (1H, CH), 4.09 s (3H, OCH3), 5.20 s (2H, OCH2), 6.26 d (1H, CH=). 13C spectrum, δ, ppm: (1R*,2R*,3S*) isomer: 14.46, 19.99, 22.46, 29.35, 33.20, 34.36, 53.66, 62.08 t (4J 4.2 Hz), 107.41 t (4J 17.4 Hz), 122.27, 126.82, 139.0 t (4J 4.2 Hz), 140.8 d (1J 248.6 Hz), 145.77 d (1J 248.6 Hz), 170.3; (1R*,2R*,3R*) isomer: 13.96, 15.37, 22.60, 27.71, 30.68, 31.36, 53.84, 62.07 t (4J 4.2 Hz), 107.41t (4J 17.4 Hz), 120.85, 124.61, 139.0 t (4J 4.2 Hz), 140.8 d (1J 248.6 Hz), 145.94 d (1J 248.6 Hz), 170.7. Mass spectrum: m/z 415 [M]+. Found, %: C 49.05; H 3.64; F 18.57; Cl 17.34. C17H16F4CL2O3. Calculated, %: C 49.18; H 3.88; F 18.30; Cl 17.08. M 415.21.
3-(2,2-Dichloroethenyl)-2-ethyl-2-propylcyclopropane acid, 4-methoxy-2,3,5,6-tetrafluorobenzyl ester (IId). Yield 80% (56% after column separation), the product contained 97% of the main substance. IR spectrum, ν, cm–1: 1735 (C=O ester), 1620 (C=C)., NMR spectrum, δ, ppm: (1R*,2R*,3S*) isomer: 0.84 t (3H, CH3), 0.87 t (3H, CH3), 1.20 m (1H, CH2), 1.36 m (2H, CH2), 1.49 m (1H, CH2), 1.66 m (2H, CH2), 1.79 d (1H, CH), 2.03 dd (1H, CH), 4.10 s (3н, OCH3), 5.15 s (2H, OCH2), 6.30 d (1H, CH=); (1R*2S*3S*) isomer: 0.84 t, (3H, CH3), 0.87 t (3H, CH3), 1.24 m (1H, CH2), 1.36 m (2H, CH2), 1.60 m (1H, CH2), 1.60 m (2H, CH2), 1.79 d (1H, CH), 2.03 dd (1H, CH), 4,08 s (CH, OCH3), 5.20 s (2H, OCH2), 6.38 d (1H, CH=). 13C spectrum, δ, ppm: (1R*,2R*,3S*) isomer: 10.20, 13.99, 17.56, 19.24, 30.64, 32.14, 36.97, 39.78, 53.40, 62.06 t (4J 4.2 Hz), 107.69 t (2J 17.4 Hz), 120.72, 124.38, 139.0 t (2J 4.2 Hz), 140.72 d (1J 248.6 Hz), 145.86 d (1J 248.6 Hz), 170.0; (1R*,2S*,3S*) isomer: 10.22, 13.99, 19.11, 26.11, 30.77, 31.07, 32.57, 36.76, 53.84, 62.06 t (4J 4.2 Hz), 107.69 t (2J 17.4 Hz), 120.66, 124.49, 139.0 t (4J 4.2 Hz), 140.72 d (1J 248.6 Hz), 145.86 d (1J 248.6 Hz), 170.0. Mass spectrum: m/z 429 [M]+. Found, %: C 50.17; H 3.84; F 17.96; Cl 16.73; C18H18F4Cl2O3. Calculated, %: C 50.37; H 4.23; F 17.70; Cl 16.52. M 429.24.
3-(2,2-Dichloroethenyl)-2-benzyl-2-methylcyclopropane acid, 4-methoxy-2,3,5,6-tetrafluorobenzyl ester (IIe). Yield 75% (52% after column separation), the product contained 97% of the main substance. IR spectrum, ν, cm–1: 1735 (C=O ester ), 1620 (C=C). 1H NMR spectrum, δ, ppm: (1R*,2R*,3R *) isomer: 1.22 s (3H, CH3), 2.04 d (1H, CH), 2.28 t (1H, CH), 2.73 d (1H, CH2), 2.82 d (1H, CH2), 4.10 s (3H, OCH3), 5.20 s (2H, OCH2), 6.34 d (1H, CH=), 7.20 d (2H, C6H5), 7.27 m (1H, C6H5), 7.32 m (2H, C6H5); (1R*,2R*,3S*) isomer: 1.21 s (3H, CH3), 1.98 d (1H, CH), 2.40 dd (1H, CH), 2.73 d (1H, CH2), 2.82 d (1H, CH2), 4.12 s (3H, OCH3), 5.20 s (2H, OCH2), 5.79 d (1H, CH=), 7.20 d (2H, C6H5), 7.27 m (1H, C6H5), 7.32 m (2H, C6H5). 13C spectrum, δ, ppm: (1R*,2R*,3R*) isomer: 12.91, 29.86, 30.95, 32.08, 46.86, 53.52, 62.07 t (4J 4.2 Hz), 107.41 t (2J 17.4 Hz), 121.35, 124.29, 126.8, 128.4, 129.3, 137.6, 139.01 t (2J 4.2 Hz), 140.8 d (1J 248.6 Hz), 145.94 d (1J 248.6 Hz), 169.8; (1R*,2R*,3S*) isomer: 17.33, 31.71, 32.49, 34.06, 41.79, 53.84, 62.07 t (4J 4.2 Hz), 107.41 t (2J 17.4 Hz), 122.61, 124.64, 126.7, 128.5, 128.9, 138.1, 139.0 t (2J 4.2 Hz), 140.8 d (1J 248.6 Hz), 145.94 d (1J 248.6 Hz), 170.1. Mass spectrum: m/z 429 [M]+. Found, %: C 55.15; H 3.51; F 16.23; Cl 16.03. C22H18F4Cl2O3. Calculated, %: C 55.36; H 3.80; F 15.92; Cl 14.86. M 477.29.
3-(2,2-Dichloroethenyl)-spiro[2.5]оctane acid, 4-methoxy-2,3,5,6-tetrafluorobenzyl ester (IIf). Yield 77% (55% after column separation), the product contained 96% of the main substance. IR spectrum, ν, cm–1: 1735 (C=O ester), 1620 (C=C). 1H NMR spectrum, δ, ppm: 1.36 m (2H, C6H10), 1.47 m (1H, C6H10), 1.48 m (2H, C6H10), 1.53 m (2H, C6H10), 1.60 m (2H, C6H10), 1.63 m (1H, C6H10), 1.69 m (1H, CH), 2.24 dd (1H, CH), 4.08 s (3H, OCH3), 5.17 s (2H, OCH2). 5.59 d (CH=). 13C spectrum, δ, ppm: 25.36, 25.50, 25.97, 29.93, 32.53, 32.60, 33.04, 36.86, 53.66, 62.04 t (2J 4.2 Hz), 121.87, 126.49, 107.45 t (2J 17. Hz), 139.1 t (2J 4. Hz), 140.7 (1J 248.6 Hz), 145.84 (1J 248.6 Hz), 170.35. Mass spectrum: m/z 441 [M]+. Found, %: C 51.49, H 3.93; F 17.44; Cl 16.17. C19H18F4Cl2O3. Calculated, %: C 51.72; H 4.11; F 17.22; Cl 16.07. M 441.25.
3-(2,2-Dichloroethenyl)-spiro[2.4]heptane acid, 4-methoxy-2,3,5,6-tetrafluorobenzyl ester (IIg). Yield 79% (58% after column separation), the product contained 96% of the main substance. IR spectrum, ν, cm–1 (C=O ester), 1620 (C=C). 1H NMR spectrum , δ, ppm: 1.57 m (4H, C5H8) d 1.59 (1H, CH), 1.63 m (4H, C5H8), 2.32 dd (1H, CH), 4.03 s (3H, OCH3), 5.11 s (2H, OCH2), 5.43 d (1H, CH=). 13C spectrum (CDCl3), δ, ppm: 25.74, 25.90, 31.72, 32.42, 32.63, 34.33, 39.51, 53.56, 62.04 m (2J 4.2 Hz), 107.63 m (2J 17.4 Hz), 121.51, 127.50, 139.1 m (2J 4.2 Hz), 140.7 g (1J 248.6 Hz), 145.84 q (1J 248.6 Hz), 170.67. Mass spectrum: m/z: 427 [M]+. Found, %: C 50.23; H 3.43; F 18.32; Cl 16.27. C18H16F4Cl2O3. Calculated, %: C 50.61; H 3.77; F 17.79; Cl 16.60. M 427.23.
Estimation of insecticidal activity. Experiments (screening tests in a cutoff concentration of 0.1%) were performed under laboratory conditions on standard sensitive laboratory strains of typhoid fly Musca domestica L., rice weevils Calandra oryzae L., bean aphides Aphys fabae Scop., and twospotted spider mite Tetranychus urticae Koch. To determine LC50 values the substances were tested in successively increasing concentrations and experiment insects mortality rates were determined for each concentration. All experiments were performed in triplicate.
The authors express their gratitude to the staff of All-Russian Institute of Chemical Means for Plant Protection for their assistance with conducting biological tests of the newly synthesized compounds.
References
- Laskowski, D.A., Reviews of Toxicology, 2002, vol. 174, p. 49.
- Galin, F.Z., Rakhimov, R.G., Vyrypaev, E.M., Bogdanov, M.P., Amirhanov, D.V., and Tolstikov, G.A., Russ. J. Org. Chem., 2000, vol. 36, p. 826.
- Sun Kim, S., Jun Kwack S., Da Lee R., J. Tox. Environ Health., 2005, vol. 68, p. 2277.
- Ma, J., Huang, R., Feng, L., Chai, Y., Prog. Nat. Sci., 2002, vol. 12, p. 271.
- Mori T., Iwasaki T., Sugano M., Shono Y., Matsuo N., Jap. Pat. 2003-206264., Chem. Abstr., 2003, 139:133688.
- Souda, H., Iwakura, K., EP 1547995, Chem. Abstr., 2004 140:99136.
- Ujihara, K., Mori T., Iwasaki T., Sugano M., Shono Y., Matsuo N., Biosci. Biotech. Biochem., 2004, vol. 68, p. 170.
- Brown, S.M., Gott, B.D., PCT, 2003/ 053 905. Chem. Abstr., 2003 139:85510.
- Nishii, Y., Maruyama, N., Wakasugi, K., Tanabe, Y., Bioorg. Med Chem., 2001, vol. 9, p. 33.
- Nägele, U.M., Hanack, M., Lieb. Ann., 1989, p. 847.
- Wilkerson, M. G., Norton S., J. Agric. Food Chem., 1981, vol. 29, p. 572.
- Nishii, Y., Wakimura, K., Tsuchiya, T., Nakamura, Sh., Tanabe, Y.J., Chem. Soc. Perkin Trans. 1., 1996, vol. 1, p. 1243.
- Kondo, K., US Pat. 4,999,451, RZhHim. (Abst. J. Chem.), 1992, 1492P.
- Souda, H., Iwakura, K., EP 1227077. Chem. Abstr., 2002 137:124945.
- Mirzabekova, N.S., Kuzmina, N.E., Lukashov, O I., Sokolova, N A., Golosov, S.N., Kazakov, P.V., Perlova,T.G., Potapova, V.V., Kheinman, V.A., and Ivanova G.B., Russ. J. Org. Chem., 2008, vol. 44, p. 1139.
- Mirzabekova, N.S, Kuzmina, N.E., Lukashov, O I., Sokolova, N A., Golosov, S.N., Kazakov, P.V., Perlova, T.G., Potapova, V.V., Kheinman, V.A., Ivanova, G.B., Russ. J. Org. Chem., 2009, vol. 45, p. 367.
- Nagayama, K., Kumar, A., Wuthrich, K., Ernst, R.R., J. Magn. Reson., 1980, vol. 40, p. 321.
- Bax, A., Subramanian, S., J. Magn. Reson., 1986, vol. 67, p. 565.
- Bax, A. and Davis, D.G., J. Magn. Reson., 1985, vol. 63, p. 207.
- Gar, K.A., Metody ispytaniya toksichnosti i effectivnosti insektitsidov (Methods for Testing Insecticides for Toxicity and Efficiency), Moscow: Nauka, 1963.
- Gordon, A., Ford, R., Sputnik khimika (Chemist’s Guide), Moscow: Mir, 1976.
Recommended for publication by Prof. A. Eleev
Fluorine Notes, 2013, 91, 3-4
