Received: November , 2013
Fluorine Notes, 2013, 91, 1-2
Synthesis of polyfluoroaromatic oligoimides with terminal anhydride groups — modifiers-hardeners of epoxy resins
T.A. Vaganova, S.Z. Kusov, M.M. Mitasov, E.V. Malykhin*
N. N. Vorozhtsov Novosibirsk Institute of Organic Chemistry, Siberian Branch of the Russian Academy
of Sciences, Lavrentiev Avenue 9, 630090 Novosibirsk, Russian Federation,
e-mail:
malykhin@nioch.nsc.ru
Abstract: The technique of preparation of polyfluoroaromatic oligoimides with terminal anhydride groups from oxydiphthalic anhydride and perfluorinated arylenediamines (benzene, naphthalene, and biphenyl derivatives) was elaborated. The products were characterized using FTIR, 1H and 19F NMR spectroscopy, HPLC-MS, and elemental analysis. The use of additives of the polyfluoroaromatic modifiers-hardeners was shown to improve thermal stability of epoxy-anhydride polymers.
Keywords: Polyfluoroarylenediamines, polyfluoroaromatic oligoimides, epoxy-anhydride polymers, thermal properties
Epoxy-anhydride polymers, which are widely used as matrixes for modern composite electrical insulating materials [1], have great potential for the targeted modification of their properties. Not only the type of resin and the nature of the curing agent can be varied, but also a huge number of organic and inorganic modifiers and fillers can be added to improve the material characteristics. Modifications of the epoxy polymers are directed, first of all, at increasing their impact resistance and fracture toughness, as well as at improving thermal stability, heat-conducting and dielectric properties. For these purposes, micro- and nanoscale inorganic dopants (oxides, carbides, and nitrides of aluminum, silicon, and boron [2-5], nanocarbon in various forms [6-8], etc.) and thermoplastics (polyesters [9], polysiloxanes [10], polyimides [11]) are used. The most promising approach appears to be based on the preparation of hybrid materials using chemical modifiers, including oligomers, which are incorporated into the polymer matrix when cured [12-14]. For example, addition of amino- and carboxyl-terminated butadiene-acrylonitrile rubbers to nanofilled epoxy composites provides fivefold increase of the fracture energy [12]. Preparation of epoxy-benzoxazine hybrids contributes to significant enhance of the flexural modulus and strength [14]. Embedding of polyfluoroalkyl-containing oxiranes in epoxy polymer matrix enhances its hydrophobicity and heat resistance of the resulting polymers [13].
We consider polyfluoroaromatic oligoimides to be promising modifiers-hardeners of epoxy resins. Highly fluorinated aromatic polyimides possess low dielectric constant and water absorption, high thermal and thermooxidative stability, and good adhesion to reinforcing materials, form thin durable coatings [15]. It can be suggested that oligoimide additives will improve a set of required properties of the epoxy matrixes.
The aims of the present work are the synthesis and characterization of anhydride terminated oligoimides based on polufluorinated diamines with various aromatic frameworks and testing them as dopants for epoxy compounds.
Results and discission
Recently we have elaborated a convenient method for aminodefluorination of polyfluoroarenes by anhydrous ammonia used as a reagent/medium system [16-18]. Individual diamines and their isomer mixtures thus obtained were successfully applied as monomers for highly fluorinated polyimides [19]. In so doing, polyimide prepared from the inexpensive mixture of isomeric hexafluoro-2,7- and 2,6-naphthylenediamines possesses characteristics comparable with those of the structurally uniform polyimide. In view of this, as diamine components for the modifiers, products of the direct bis-aminodefluorination of base perfluoroarenes were choose: the mixture of isomeric tetrafluoro-1,3- and -1,4-phenylenediamines in the ratio 5:1 (4FPDA) was used for modifier M-1, the mixture of hexafluoro-2,7- and -2,6-naphthylenediamines in the ratio 4:1 (4FNDA) - for modifier M-2, and octafluorobenzidine (8FBDA) - for modifier M-3 (Scheme 1). As a dianhydride component, commercially available 4,4'-oxydiphthalic anhydride (ODPA) was used.
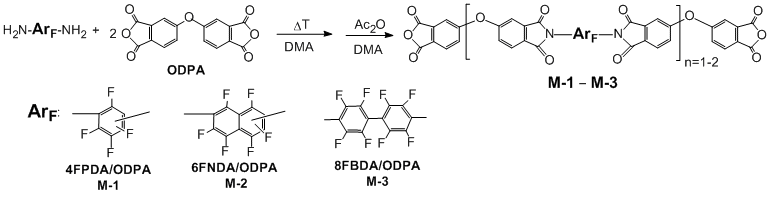
Scheme 1. Synthesis and structures of the polyfluorinated oligoimide modifiers.
To carry out the condensation, polyfluoroarylenediamine was added in small portions to a solution of 2 equivalents ODPA in dried DMA, and the mixture was stirred at 70-80 В°C until starting material was consumed; degree of the conversion was monitored using 19F NMR. Depending on the diamine reactivity, the reaction time ranged from 15 to 25 hours. Acetic anhydride was used for the chemical cyclodehydration of the intermediate amic acids; after completion of the reaction, solvents were distilled off under reduced pressure at 150 В°C.
Oligoimides M-1 and M-2 derived from the mixtures of perfluorinated phenylene- and naphthylendiamines, respectively, are soluble in polar organic solvents (DMA, N-methylpyrrolidone, DMSO), including low-boiling chloroform and acetone. Oligomer M-3 based on perfluorobiphenyl becomes practically insoluble after complete imidization.
Soluble oligoimide modifiers were characterized using FTIR, 1H and 19F NMR spectroscopy, HPLC-MS, and elemental analysis. The used ratio of diamine and dianhydride (1:2, respectively) and sequence of mixing the reagents provide preferential formation of diimides with a central perfluoroaromatic moiety and terminal anhydride functions. Besides, compounds with n=2 (see Scheme 1) were registered by HPLC-MS as minor components. Ratio of the fluorinated and non-fluorinated fragments in the products was determined by NMR using 4,4'-dimethoxyoctafluorobiphenyl as an internal standard. In the 19F NMR spectra of the  oligoimides there are signals of fluorine atoms in the diimide fragment, in the FTIR spectra – absorption bands of the carbonyl groups in the anhydride and imide fragments. Evidences of the presence of amine and amic acid fragments were not detected spectroscopically. Structure of the insoluble modifier M-3 was confirmed by FTIR spectroscopy and elemental analysis.
Effect of the polufluoroaromatic oligoimides on curing and thermal characteristics of the modified epoxy-anhydride polymers was investigated by example of the epoxy formulations composed of diglycidyl ether of bisphenol A (DGEBA), isomethyltetrahydrophthalic anhydride (IMTHPA), and modifiers M-1 - M-3 (10 parts by weight of DGEBA) (Table 1). The molar compositions were calculated taking into account a stoichiometrically balanced amount of the anhydrides sum: 0.52 eq of the curing agent (M-1,2,3 + IMTHPA) per 100 g of DGEBA (Epoxide Percentage 22.5). Oligoimides M-1 and M-2 were dissolved in IMTHPA upon heating; insoluble modifier M-3 milled to a particle size <10 Ојm was dispersed ultrasonically in IMTHPA to form a stable suspension. Curing was carried out in stepwise mode in the presence of a catalytic amount of dimethylbenzylamine (BDMA). Simultaneously, a reference sample of the epoxy-anhydride polymer (E0) containing no modifier was prepared.
Table 1. Composition and thermal properties of the modified epoxy polymers.
|
Formulation |
Composition, parts by weight |
Glass transition temperature, DSC |
Thermooxidative stability, TGA |
||||
|
|
DGEBA |
M |
IMTHPA |
BDMA |
Tg, В°C |
T(5%), В°C |
T(10%), В°C |
|
E0 |
100 |
|
86 |
1 |
121 |
345 |
377 |
|
EM-1 |
100 |
10 |
82 |
1 |
141 |
356 |
387 |
|
EM-2 |
100 |
10 |
82.4 |
1 |
135 |
348 |
373 |
|
EM-3 |
100 |
10 |
82.7 |
1 |
126 |
354 |
380 |
To control curing of the epoxy compounds, FTIR spectroscopy was used. As an example, Fig. 1 shows the spectra of EM-1 before and after curing. Spectra of the starting mixtures contain overlapping absorption bands of the anhydride carbonyls in IMTHPA and phthalic moiety of modifiers M-1,2,3 (1852 and 1778 cm-1). Disappearance of these bands after curing points to the complete conversion of the anhydride groups of modifiers, including those in the insoluble M-3. Instead of them, absorption band of ester carbonyls (1734 cm-1), formed due to the interaction of the anhydride and epoxy components, appears in the spectra of cured compounds. As a result of curing, overlapping absorption bands of the anhydride and epoxy cycles (916 and 972 cm-1) also disappear. A strong band at 1740 cm-1 in the initial spectra belongs to the imide carbonyl of the modifiers; in the spectra of cured compounds it overlaps with the ester absorption. A weak band of the imide carbonyl at 1798-1800 cm-1, which is covered by the strong band of anhydride carbonyls in the initial spectra, appears in the spectra of the cured compounds. In whole, the changes observed testify to the incorporation of the bifunctional oligoimide modifier into epoxy-anhydride matrix.
According TG/DSC analysis, the modified epoxy polymers EM-1,2,3, containing thermostable polyfluoroaromatic imide fragments, possess improved thermal characteristics in comparison to the non-modified E0 (Table 1). Modifiers M-1 and M-2, based on isomeric perfluoroarylenediamines, provide an appreciable increase of the glass transition temperature, which characterizes the heat resistance of the polymer. In addition, the oxidative stability, determined from the temperature at 5 and 10% weight loss, rises when using modifiers M-1 and M-3.
Thus, the technique of the preparation of imide-anhydride modifiers-hardeners containing various polyfluoroaromatic fragments was elaborated. These substances are shown to be promising for the preparation of the epoxy-polyimide matrixes with improved performance.
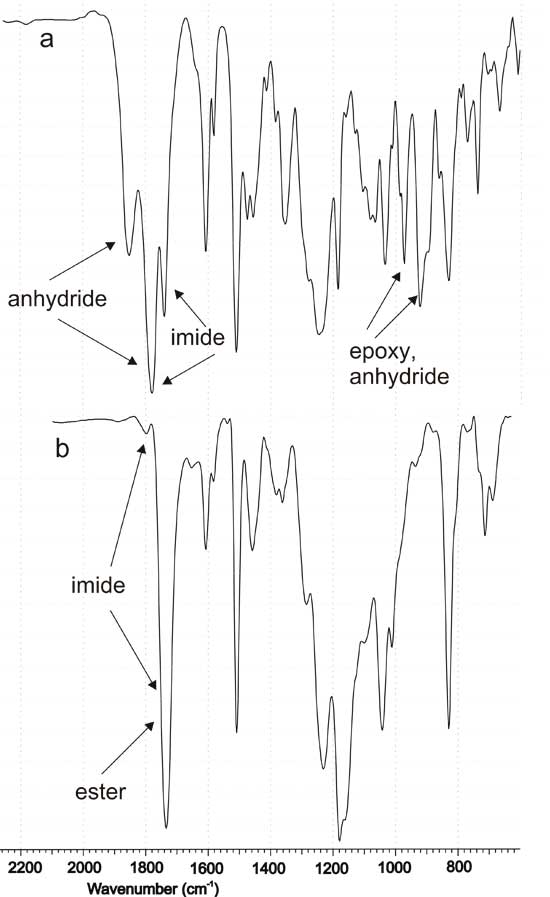
Fig. 1. FTIR spectra of the modified epoxy compound EM-1 before (a) and after (b) curing.
Experimental
1H and 19F NMR (δ, ppm) spectra were recorded on NMR spectrometer Bruker AV-300 (300.13 and 282.36 MHz, respectively) using residual proton signals of the deuterated solvent and C6F6 (0 ppm) as internal standard. Fourier transform infrared (FTIR) spectra were measured on Bruker Vector-22 instrument in the ATR mode. HPLC-MS analysis was performed using liquid chromatograph Agilent 1200 and hybrid quadrupole-time-of-flight mass spectrometer with an electrospray interface micrOTOF-Q, Bruker Daltonics. The API-ES mass spectra were recorded for m/z 100–3000 in the negative scan mode. Nitrogen was used as a drying gas at 220 °C and at flow of 4 L min−1. Thermogravimetry (TG) and differential scanning calorimetry (DSC) were performed on NETZSCH STA 409 instrument with heating rate 10 °C min-1 under flowing He or He/O2 mixture (8:2, v/v).
The mixture of isomeric tetrafluoro-1,3- and -1,4-phenylenediamines in the ratio 5:1 (4FPDA) was prepared as described in [17], the mixture of isomeric hexafluoro-2,7- and -2,6-naphthylenediamines in the ratio 4:1 (4FNDA) – as described in [16], octafluorobenzidine (8FBDA) – as described in [17], N,N-dimethylbenzylamine – as described in [20]. Dimethylacetamide (DMA) was purified by distillation over P2O5 under reduced pressure and stored over 3E molecular sieve, residual moisture <0.02%. 4,4’-Oxydiphthalic anhydride (ODPA) was purified by crystallization from the mixture of acetic anhydride and acetic acid. Commercial reagents: epoxy resin D.E.R. 330, Dow Chemical Company, Epoxide Percentage 22.5 % (DGEBA); isomethyltetrahydrophthalic anhydride, Technical Standard 8.103149-85 (IMTHFA); and acetic anhydride.
Synthesis of the modifiers (a typical procedure). A round-bottomed flask equipped with a magnetic stirrer bar and downtake condenser was charged with ODPA (0.2 mol) and DMA (250 mL), the mixture was stirred at 70-80 В°C to dissolve the anhydride, and polyfluoroarylenediamine (0.1 mol) was added in small portions. The solution obtained was kept at this temperature until starting material was consumed (15-25 h); the degree of conversion was monitored using 19F NMR. Acetic anhydride was added (1.4 mol) and the mixture was kept under the same conditions for 5-6 h. Solvents were distilled off under reduced pressure and the pasty mass obtained was dried in a vacuum oven (10-15 mm Hg) at 150-180 В°C.
Modifier M-1 (4FPDA/ODPA). Yield 95 %. Melting range 142-148 °C. FTIR: 3076, 3105 (Car-H), 1850, 1776, 1734 (C=O in anhydride and imide cycles). 1H NMR (ac-d6): 7.76, 7.81, 8.16 (all multiplets of the equal intensity). 19F NMR (ac-d6): 1,3-disubstituted tetrafluorophenylene fragment – 1.9 (m, 1F, F5), 30.2 (m, 2F, F4, F6), 40.2 (m, 1F, F2); 1,4-disubstituted tetrafluorophenylene fragment – 21.3 (m, 4F, F2, F3, F5, F6). HPLC-MS: the major peak [M+OH]- 781.06, C38H12F4N2O12+OH, calcd 781.50, the minor peak [M+OH]- 1235.03, C60H18F8N4O17+OH, calcd 1235.79. Elemental: found C 59.0, H 1.54, N 3.05, F 8.94; calcd for C38H12F4N2O12 C 59.7, H 1.57, N 3.66, F 9.95.
Modifier M-2 (6FNDA/ODPA). Yield 96 %. Melting range 162-170 °C. FTIR: 3076, 3105 (Car H), 1852, 1776, 1734 (C=O in anhydride and imide cycles). 1H NMR (ac-d6+DMSO-d6): 7.65, 7.80, 8.16 (all multiplets of the equal intensity). 19F NMR (ac-d6+DMSO-d6): 2,7-disubstituted hexafluoronaphthylene fragment – 16.0 (m, 2F, F4, F5), 25.5 (m, 2F, F3, F6), 43.4 (m, 2F, F1, F8); 2,6-disubstituted hexafluoronaphthylene fragment – 17.6 (m, 2F, F4, F8), 23.0 (m, 2F, F3, F7), 41.6 (m, 2F, F1, F5). HPLC-MS: the major peak [M+OH]- 867.04, C42H12F6N2O12+OH, calcd 867.54, the minor peak [M+OH]- 1407.00, C68H18F12N4O17+OH, calcd 1407.87. Elemental: found C 59.2, H 1.33, N 2.97, F 13.2; calcd for C42H12F6N2O12 C 59.3, H 1.41, N 3.29, F 13.4.
Modifier M-3 (8FBDA/ODPA). Yield 98 %. Does not melt up to 300 В°C. FTIR: 3078, 3105 (Car-H), 1852, 1778, 1734 (C=O in anhydride and imide cycles). Elemental: found C 57.5, H 1.23, N 2.73, F 16.9; calcd for M C44H12F8N2O12: C 57.9, H 1.32, N 3.07, F 16.7.
Preparation of the modified epoxy compounds. Modifier M-1 or M-2 was dissolved in IMTHPA under stirring and heating up to 100-120 В°C, the solution was cooled to the ambient temperature. The insoluble modifier M-3 ground to a particle size of 5-7 Ојm in a ball vibromill was dispersed in IMTHPA by ultrasonication for 20 min. To the solution or suspension obtained, DGEBA heated to 50 В°C was added, the mixture was stirred and BDMA was added. Compositions of the samples are given in Table 1. The mixture prepared was ultrasonicated for 4-6 min, placed in a silicon mold, and degassed in a vacuum oven for 0.5 h at 40 В°C. Curing was carried out in a stepwise mode: 100 В°C/2 h + 160 В°C/10 h; heating rate to the cure temperature was 2 В°C min-1.
The work was financially supported by the Siberian Branch of the Russian Academy of Sciences,
grant 97 (fundamental affiliate research 2012-2014).
Authors thank Dr. I.K. Shundrina
for the TG/DSC analysis of epoxy polymers, Dr. V.G. Vasil’ev for the HPLC-MS analysis of
the oligoimides.
References
- Mikhailin, Yu.A. Termoustoichivye polimery i polimernye materialy // SPb: Professiya, 2006. 624 s.; Petrie, E.M. Epoxy adhesive formulations // New-York: McGraw-Hill; 2006. 534 p.
- Kochetov, R., Andritsch, T., Morshuis, P.H.F., Smit, J.J. Dielectric response and thermal conductivity of epoxy resin filled with nanoalumina particles of different size in О±, Оі and Оґ phase // Electrical Insulation and Dielectric Phenomena (CEIDP), 2010 Annual Report Conference, 1-4, DOI 10.1109/CEIDP.2010.5723963.
- Li, H., Zhang, Z., Ma, X., Hu, M., Wang, X., Fan, P. Synthesis and characterization of epoxy resin modified with nano-SiO2 and Оі-glycidoxypropyltrimethoxy silane // Surf. Coat. Technol. 2007, vol. 201, N 9-11, p. 5269-5272.
- Yung, K. C., Liem, H. Enhanced Thermal Conductivity of Boron Nitride Epoxy-Matrix Composite Through Multi-Modal Particle Size Mixing // J. Appl. Polym. Sci. 2007, vol. 106, N 6, p. 3587-3591.
- Fua, J.-F., Shia, L.-Y., Zhong, Q.-D., Chena, Y., Chen, L.-Y. Thermally conductive and electrically insulative nanocomposites based on hyperbranched epoxy and nano-Al2O3 particles modified epoxy resin // Polym. Adv. Technol. 2011, vol. 22, N 6, p. 1032-1041.
- Geng, Y., Liu, M.Y., Li, J., Shi, X.M., Kim, J.K. Effects of surfactant treatment on mechanical and electrical properties of CNT/epoxy nanocomposites // Composites: Part A 2008, vol. 39, N 12, p. 1876-1883.
- Lee, Y.S., Cho, T.H., Lee, B.K., Rho, J.S., An, K.H., Lee, Y.H. Surface properties of fluorinated single-walled carbon nanotubes // J. Fluorine Chem. 2003, vol. 120, N 2, p. 99-104.
- Gabriel, G., Sauthier, G., Fraxedas, J., Moreno-Manas, M., Martinez, M.T. Miravitlles, C. Preparation and characterization of single-walled carbon nanotubes functionalized with amines // Carbon 2006, vol. 44, N 10, p. 1891-1897.
- Flores, M., FernГЎndez-Francos, X., Ferrando, F., Ramis, X., Serra, A. Efficient impact resistance improvement of epoxy/anhydride thermosets by adding hyperbranched polyesters partially modified with undecenoyl chains // Polymer 2012, vol. 53, N 23, p. 5232-5241; Foix, D., Ramis, X., Ferrandoc, F., Serra, A. Improvement of epoxy thermosets usinga thiol-ene based polyester hyperbranched polymer as modifier // Polym. Int. 2012, vol. 61, N 5, p. 727-734.
- Rey, L., Poisson, N., Maazouz, A., Sautereau, H. Enhancement of crack propagation resistance in epoxy resins by introducing poly(dimethylsiloxane) particles // J. Mater. Sci. 1999, vol. 34, N 8, p. 1775-1781.
- Hay, J.N., Woodfine, B., Davies, M. Toughening of epoxy resins by polyimides synthesized from bisanilines // High Perform. Polym. 1996, vol. 8, N 1, p. 35-56; Gaw, K.O., Kakimoto, M. Polyimide-Epoxy Composites // Adv. Polym. Sci. 1999, vol. 140, p. 107-136.
- Hsieh, T. H., Kinloch, A. J., Masania, K., Sohn Lee, J., Taylor, A. C., Sprenger, S. // The toughness of epoxy polymers and fibre composites modified with rubber microparticles and silica nanoparticles // J. Mater. Sci. 2010, vol. 45, N 3, p. 1193-1210.
- Fedoseev, M. S., Derzhavinskaya, L. F., Karmanov, V. I., Bazhin, D. N., Zapevalov, A. Ya., Gorbunova, T. I., Saloutin, V. I. Synthesis and Properties of Epoxy-Anhydride Polymers Modified with Polyfluorolakyl-Substituted Oxiranes in the Course of Curing // Russ. J. Appl. Chem. 2010, vol. 83, No. 4, p. 723-727; Fedoseev, M. S., Glushkov, V. A., Derzhavinskaya, L. F., Krainova, G. F., Tiunova, T. G. Synthesis and curing of epoxy-anhydride polymers modified with an oxirane of the tetrahydroquinoline series // Russ. J. Appl. Chem. 2011, vol. 84, No. 9, p. 1596-1599.
- Grishchuk, S., Mbhele, Z., Schmitt, S., Karger-Kocsis, J. Structure, thermal and fracture mechanical properties of benzoxazine-modified amine-cured DGEBA epoxy resins // eXPRESS Polym. Let. 2011, vol. 5, N.3, p. 273-282.
- Hougham, G. Fluorine-Containing Polyimides // G. Hougham (Ed.), Fluoropolymers 2: Properties, Kluwer Academic/Plenum Publishers, New York, 1999, p. 233-276.
- Vaganova, T.A., Kusov, S.Z., Rodionov, V.I., Shundrina, I.K., Sal'nikov, G.E., Mamatyuk, V.I., Malykhin, E.V. Amination of octafluoronaphthalene in liquid ammonia. 2,6- and 2,7-Diaminohexafluoronaphthalenes selective preparation // J. Fluor. Chem. 2008, vol. 129, N 4, p. 253-260.
- Vaganova, T.A., Kusov, S.Z., Rodionov, V.I., Shundrina, I.K., Malykhin, E.V. Selective mono- and diamination of polyf luorinated benzenes and pyridines with liquid ammonia // Russ. Chem. Bull., Int. Ed. 2007, vol. 56, N 11, p. 2239-2246.
- Vaganova, T.A., Kusov, S.Z., Rodionov, V.I., Shundrina, I.K., Malykhin, E.V. Direct di- and triamination of polyfluoropyridines in anhydrous ammonia // J. Fluor. Chem. 2009, vol. 130, N 5, p. 461-465.
- Malykhin, E.V., Vaganova, T.A., Shundrina, I.K., Kusov, S.Z., Rodionov, V.I., Karpova, E.V. Polyimides of AA/BB- and AB-Types Based on New Perfluorinated Monomers // Chem. Sustain. Development 2011, vol. 19, N 6, p. 661-668; Shundrina, I.K., Vaganova, T.A., Kusov, S.Z., Rodionov, V.I., Karpova, E.V., Koval, V.V., Gerasimova, Yu.V., Malykhin, E.V. Synthesis and characterization of polyimides based on novel isomeric perfluorinated naphthylenediamines // J. Fluor. Chem. 2009, vol. 130, N 8, p. 733-741.
- Tayama, E., Kimura, H. Asymmetric Sommelet–Hauser Rearrangement of N-Benzylic Ammonium Salts // Angew. Chem., Int. Ed. 2007, vol. 46, N 46, p. 8869-8871.
Recommended for publication by Prof. V. Platonov
Fluorine Notes, 2013, 91, 1-2
