Received: November , 2012
Fluorine Notes, 2013, 89, 1-2
The distinctive features of carboxymethylcellulose sodium salt surface polyfluoroalkyl-modification
A.I.Rahimov, O.V.Vostrikova, E.V.Petrosyan
*Russia, Volgograd State Technical University, 400131, Volgograd, Lenina ave. 28
e-mail: organic@vstu.ru
Abstract: It is shown that the surface polyfluoroalkyl modification of carboxymethylcellulose sodium salt with octafluoropentylchlorosulfite resulted in the formation of octafluoropentyl esters and ethers with total content of fluorine 6.67%, and allows considerable reduction of roughness on the surface of solids sized up to 1.4 µm.
Keywords: carboxymethylcellulose sodium salt, octafluoropentylchlorosulfite, esters and ethers, roughness of surface.
Research of heterophase polyfluoroalkylation of surfaces that involve hydroxyl and carboxy groups was exemplified by the modification of detonation nanodiamonds [1]. It was a multistep modification process that included the surface treatment with thionylchloride, after that the chloroanhydride groups and chlorine atoms thus formed reacted with sodium alcoholate of perfluorobutylmethanol.
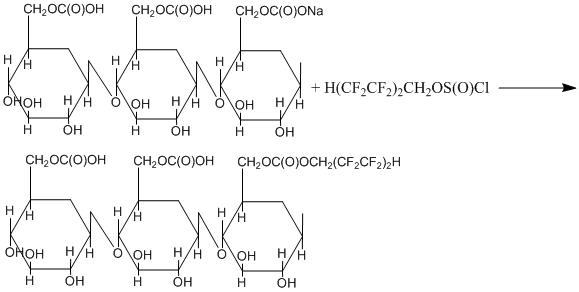
We carried out the modification of carboxy- and hydroxyl- groups on the surface of 50-100 µm solid particles of carboxymethylcellulose sodium salt via their reaction with octafluoropentylchlorosulfite according to our earlier disclosed techniques [2-8].
The study on the modified carboxymethylcellulose structure by Fourier-transform spectrometry has shown that polyfluoroalkylchlorosulfite reacted with Na-carboxylate and HO-groups resulting in polyfluoroalkyl ethers and esters with total fluorine content of 6.67% according to the schedule as follows:
Octafluoropentyl ester groups were identified in IR spectra (Fig. 1, table 1) by the carboxy group absorption band (observed at 1720 cm-1), assigned to the ester substituent valence vibrations, while a very intensive band at 1213 cm-1 evidenced the presence of a polyfluoroalkyl group. It is very probable that the band at 1139 cm-1 belongs to >C-O-CH2(CF2)4 -group The said valence vibrations are not present in the IR spectra of original carboxymethylcellulose.
Table 1. IR absorption bands in the spectra of original carboxymethylcellulose sodium salt and in those of carboxymethylcellulose octafluoropentyl ethers and esters.
|
# |
Groups |
IR absorption bands, cm-1 |
|
|
Na-CMC |
F-CMC |
||
|
1 |
HO, COOH |
1284,1310,1381,1421, 3500-3600 |
1390, 1412, 3400-3600 |
|
2 |
-COONa |
1594 |
1601 |
|
3 |
Cyclo- CH2-O-CH2- |
1064 |
1064 |
|
4 |
-C(O)O-CH2(CF2)4H |
- |
1720 |
|
5 |
-C-O-CH2(CF2)4H |
- |
1139 |
|
6 |
-(CF2)4- |
- |
1213 |
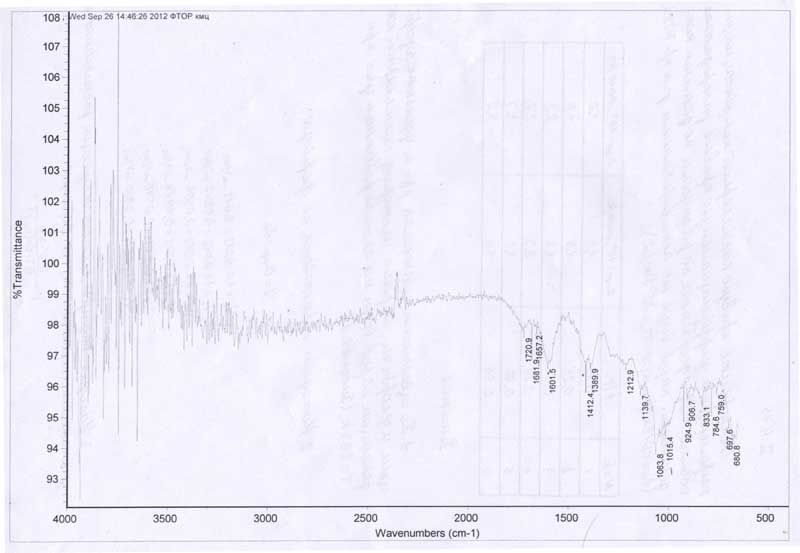
Figure 1. Fourier-transform spectrum of modified carboxymethylcellulose
The surface of modified carboxymethylcellulose sodium salt was studied by the method of atomic force microscopy with the help of “Solver Pro” probe atomic force scanning microscope, and compared with the surface of original carboxymethylcellulose sodium salt (Fig.2). The most important conclusion derived from the obtained results is that roughness of the modified carboxymethylcellulose surface reduced to 1.4 µm (while in original carboxymethylcellulose sample it exceeded 3 µm), the fact is attributable to the introduction of polyfluoroalkyl chains (CF2)4 belonging to the ester and ether groups. The said groups are known to provide hydrophobicity, decreasing the probability of associative inter- and intra-molecular interactions, reducing therefore the surface “roughness”.
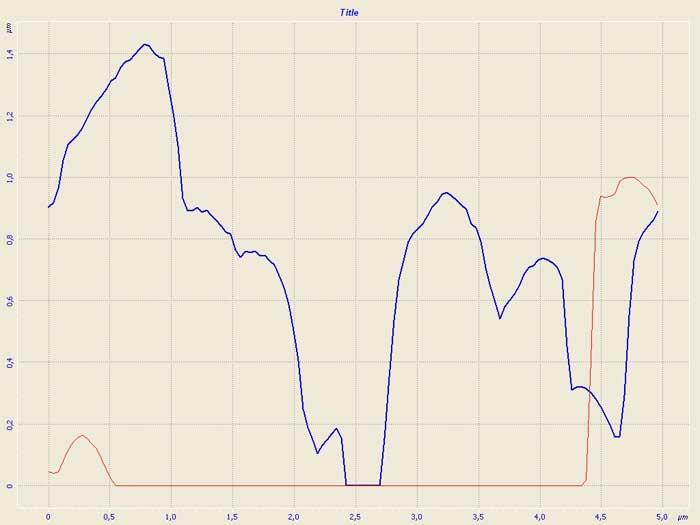
Figure 2. Diagram of roughness on the surface of modified carboxymethylcellulose.
Figure 2 presents the variation in the surface roughness within 5mcm sector. This variation reaches its maximal value at about 1.4 µm. This observation is confirmed by three-dimension image (Fig. 3).
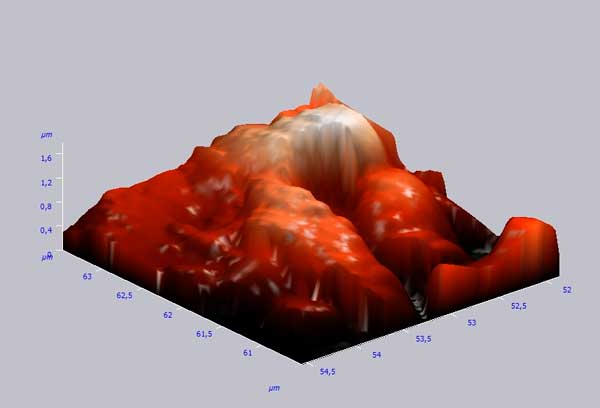
Figure 3. Three-dimensional image of the modified carboxymethylcellulose surface.
Therefore, polyfluoroalkylation of carboxymethylcellulose sodium salt by octafluoropentylchlorosulfite makes it possible to reduce its surface “roughness” and add hydrophobicity.
References
2. Rahimov, A.I. Novyj metod sinteza poliftorirovannyh prostyh ehfirov // Zhurnal organicheskojkhimii / A. I. Rahimov, A. V. Nalesnaya, O. V. Vostrikova. - 2004. - T. 74. , vyp. 4. -s. 1121.
3. Rahimov, A. I. Reakciya poliftoralkilhlorsul'fitov s benzilovymi spirtami // Zhurnal obshchejkhimii / A. I. Rahimov, R. V. Fisechko. - 2007. - T. 77, vyp. 10. -s. 1750-1751.
4. Rahimov, A.I. Novyj metod sinteza poliftoralkilciklogeksilovyh ehfirov // Zhurnal obshchejkhimii / A. I. Rahimov, R. V. Fisechko. - 2008. - T. 78, vyp. 2. -s.338.
5. Rahimov, A.I. Osobennosti kataliza reakcii poliftoralkil-hlorsul'fitov s predel'nymi odnoatomnymi spirtami // Zhurnal obshchejkhimii / A. I. Rahimov, A. V. Nalesnaya, R. V. Fisechko. - 2008. - T. 78, vyp. 11. -s. 1842-1848.
6. Patent RU 2346926, MPK C 07 C43/192, C 07 C43/12, C 07 C43/225, C 07C 43/174. Sposob polucheniya prostyh poliftoralkilovyh ehfirov / A.I. Rahimov, A.V. Nalesnaya, R.V. Fisechko; GOU VPO VolgGTU. - 2009.
7. Rahimov, A.I. Sintez poliftoralkilhlorsul'fitov i novye reakcii s ih uchastiem // Zhurnal obshchej khimii - 2010. - T. 80, vyp. 8. - s. 1622-1641.
8. Rahimov, A.I. Sintez allilovyh ehfirov poliftorirovannyh spirtov / A.I. Rahimov, A.V. Miroshnichenko, Zyong Fyong Thao Do // Izvestiya VolgGTU. Seriya «Khimiya i tekhnologiya ehlementoorganicheskih monomerov i polimernyh materialov». Vyp. 7: mezhvuz. sb. nauch. st. / VolgGTU. - Volgograd, 2010. - # 2. - s. 47-49.
Recommended for publication by Prof. Alexander I. Rakhimov
Fluorine Notes, 2013, 89, 1-2
