Received: March, 2013
Fluorine Notes, 2013, 88, 7-8
The solvent effects on co-polymerisation of tetrafluoroethylene with perfluoro(-3,6-dioxa-4-methyl-7-octene)sulfonylfluoride
A.S. Odinokov, O.S. Bazanova, L.F. Sokolov, V.G. Barabanov, B.N.Maximov
FSUE В«Russian Scientific Center В«Applied ChemistryВ», 193232, Russia, St. Petersburg, Krilenko ave. 26A
e-mail: vbarabanov@rscac.spb.ru
Abstract: A search for solvents is conducted and solvent effects are investigated on the initiator (perfluorodiacylperoxide) destruction, and on the composition of copolymer resulting from the solution radical co-polymerisation of tetrafluoroethylene with perfluoro(-3,6-dioxa-4-methyl-7-octene)sulfonylfluoride.
Keywords: PSVE, perfluoro(3,6-dioxa-4-methyl-7-octene)sulfonylfluoride, tetrafluoroethylene.
Radical co-polymerisation of tetrafluoroethylene (TFE) with perfluoro(-3,6-dioxa-4-methyl-7-octene)sulfonylfluoride (PSVE, FC-141) occurs according to the diagram as follows resulting in the formation of perfluorinated copolymer F-4SF:
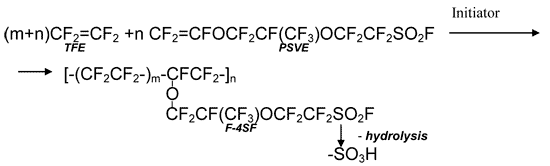
There are a lot of methods for the manufacture of F-4SF copolymer. The most efficient is solution method in perfluorinated solvent environment. In publications, including patents, 1,1,2-trifluoro-1,2,2-trichloroethane (CFC113) is the most often mentioned solvent for PSVE/TFE co-polymerisation. Unfortunately, the usage of this solvent is now ruled out due to its high ozone-depleting potential (ODP = 0.8). The list of solvents applicable as CFC113 substituents is small. They must meet the requirements as follows:
- high solvency of TFE and PSVE in the chosen solvent; that is why such substituents were elected from chlorofluorocarbons;
- the solvent must be inert in the radical co-polymerisation process, neither participate in the said polymerisation process nor perform as a chain-transfer agent;
- acceptable physico-chemical properties for development of the copolymer manufacture process based on the chosen solvent; low toxicity, fire-and-explosion safety, low boiling temperature;
- market availability, i.e. possibility to purchase the solvent in amounts necessary for the production creation;
- cost effectiveness; low price.
Reasoning from the above requirements the following substances were chosen for tests:
- RC316 (1,2-dichlorohexafluorocyclobutane), the most close analogue of CFC113, that results from the cyclo-dimerisation of trifluorochloroethylene as a mixture of a few isomers. Due to exhaustive halogenation of RC316 its inertness in radical polymerisation is comparative to that of CFC113. RC316 is non-combustible and non-explosive. The substance is ozone-depleting, but yet non-regulated by the Montreal Protocol. Its price is comparable to that of CFC113.
- MD46 (perfluoromethyldiethylamine) is a by-product in perfluorotriethylamine production. It is the less-common of the solvents under consideration. The substance is highly resistant to radical processes, and enables to achieve the best strength and proton conductivity. The estimated price is given below.
- PSVE. The method of co-polymerisation using the ether monomer PSVE for solvent is also mentioned in literature [1, 2]. However, with this method it is very difficult to provide constant composition of the copolymer product; and the monomer losses during its regeneration increase.В The main properties of considered solvents are shown below in Table 1.
Table 1. Characteristics of solvents for solution polymerisation.
|
Solvent |
Chemical formula |
Resistance to radicals |
MAC, mg/m3 |
Boiling temperature, oC |
Availability |
Price, ruble/kg |
|
RC316 |
C4F6Cl2 |
Middle |
10 |
59 |
Low |
2000 |
|
MD46 |
C5F13N |
High |
500 |
46 |
Low |
9000 |
|
PSVE |
C7F14O4S |
_ |
50 |
-6 |
middle |
20000 |
The solvent impact on the destruction of the co-polymerisation initiator was studied. Bis-(perfluorocyclohexanoyl)peroxide (DAP-C) was used for initiator; the substance synthesis is based on the interaction between perfluorocyclohexane carbonyl fluoride (PFCHC) and Na2O2, formed by the reaction between NaOH and hydrogen peroxide.
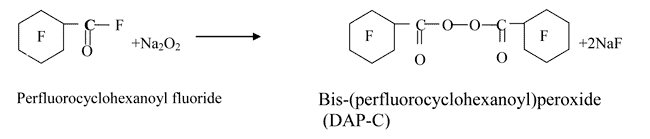
Thermal decomposition of DAP-C in a solvent under study was conducted in a sealed glass sample-tube placed into an ultra-thermostat. The temperature was being kept constant with accuracy 0.1В°C. The peroxide concentration was determined by iodometric titration in equal time intervals. A graph of peroxide concentration dependence on its decay-period was constructed, and, hence, the peroxide half-decay period was determined. The results are shown in Table 2.
Table 2. DAP-C thermal decomposition in various solvents.
|
Solvent |
DAP-C half-decay period |
||
|
30В°C |
40В°C |
50В°C |
|
|
CFC113 |
~49 hour |
13 hour 45 min |
3 hour 47 min |
|
MD-46 |
~41 hour |
10 hour 15 min |
2 hour 30 min |
|
RC316 |
~44 hour |
11 hour 40 min |
3 hour 05 min |
From Table 1 one may see that DAP-C half-decay periods in MD-46 and RC316 are very close, and consequently, from this point of view, both solvents may be considered as acceptable CFC113 substituents.
TFE solubility in PSVE and in solvents was studied within the working temperature interval.
TFE solubility was studied in a 0.2 dm3 reactor equipped with a stirrer, jacket, thermocouple (accuracy 0.1В°C), and manometer (accuracy 0.1atm). 150g of the solvent under study was charged into the preliminary evacuated reactor at room temperature, and TFE was fed to total pressure 2 atmg. Then we started raising the reactor temperature by step of 10В°C; at each step the procedure stopped for 1 hour to achieve equilibrium, and recorded both temperature and pressure values. TFE gas-phase concentration was calculated from the reactor pressure and reference density values. Our calculations took into account the solvent specific vapour pressure and its amount turned into gas phase. TFE liquid-phase concentration was determined by subtraction of gas-phase TFE amount from that charged into the reactor.
The solvent Henry constants are graphically shown in Figure 1.
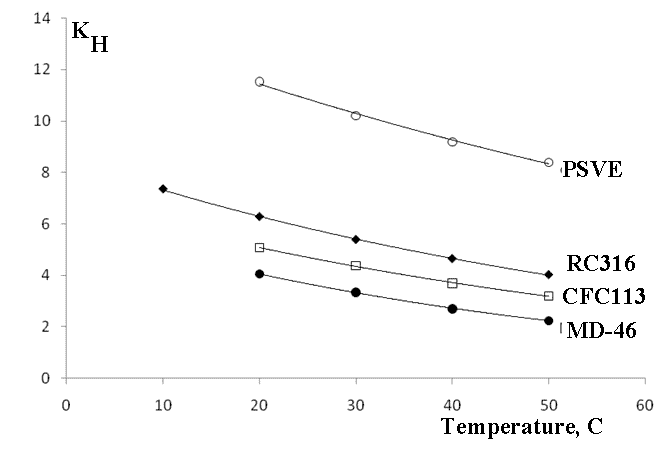
KH - Henry constant, TFE concentration in liquid : TFE concentration in gas phase.
Figure 1. TFE solubility in PSVE, RC316, MD46, and CFC113.
We further studied the solvent dependence of the co-polymer composition. In our calculations of TFE/PSVE co-polymer composition were applied the terminal link model developed by Frank R. Mayo and Frederick M. Lewis.
According to that model the copolymer composition follows the formula:
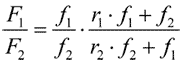
Here F1 and F2 are mole shares of TFE and PSVE monomers in the copolymer; f1 and f2 those in the monomer blend; r1 and r2 are TFE and PSVE В co-polymerisation constants.
The graphically obtained results are shown in Figure 2.
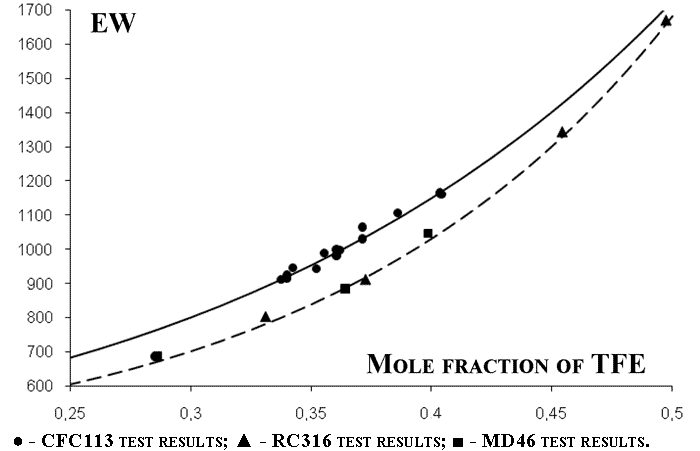
EW- equivalent weightВ
Figure 2. The co-polymer composition dependence on the TFE/PSVE mole ratio in the reaction blend.
We determined the constants at PSVE conversion values of 15-20%. For the polymerisation process conducted in CFC113 environment the constant values were rTFE= 23.3; rPSVE=0.9. In cases of MD46 and RC316 those values were rTFE= 73; and rPSVE=5, correspondingly.
Conclusions
- The half-decay periods for DAP-C synthesized in MD-46 or RC-316 solvents are very close.
- From our estimation of melt flow indices one may conclude that the molecular weights of copolymers prepared in MD46, RC316, and PSVE solvents exceed that of produced in CFC113.
- The TFE/PSVEco-polymerisation constants calculated for MD46 and RC316 solvents differ considerably from those for CFC113, suggesting that CFC113 participates in the co-polymerisation process.
- Any of considered solvents may perform as CFC113 substituent in this process. The obtained constant values allow high-accuracy prognostication of the co-polymer composition when the polymerization is conducted in RC316 or MD46.
References
- Pat. 4116888 US, US Cl 521/28. Process for producing fluorinated copolymer having ion-exchange groups.
- Pat. 4349650 US, US Cl 526/243. Polyfluoroallyloxy compounds, their preparation and copolymers therefrom.
The study was conducted within the framework of State Contracts # 12411.1007499.09.174 of 21.09. 2012 and # 11.519.11.6024 of 25.10.2011.
Recommended for publication by V. Kornilov
Fluorine Notes, 2013, 88, 7-8
