Received: March, 2013
Fluorine Notes, 2013, 88, 5-6
Kinetics of co-polymerisation of tetrafluoroethylene with perfluoro(3,6-dioxa-4-methyl-7-octene)sulfonylfluoride
S. Odinokov, O. S. Bazanova, L. F. Sokolov, V. G. Barabanov
FSUE В«Russian Scientific Center В«Applied ChemistryВ», 193232, Russia, St. Petersburg, Krilenko ave. 26A
e-mail: vbarabanov@rscac.spb.ru
Abstract: Both temperature and monomer concentration effects on the kinetics of solution radical co-polymerisation of tetrafluoroethylene with perfluoro(3,6-dioxa-4-methyl-7-octene)sulfonylfluoride were studied.
Keywords: PSVE, perfluoro(3,6-dioxa-4-methyl-7-octene)sulfonylfluoride, tetrafluoroethylene.
Co-polymerisation of tetrafluoroethylene (TFE) and perfluoro(-3,6-dioxa-4-methyl-7-octene)sulfonylfluoride (PSVE, FC-141) results in the formation of perfluorinated co-polymer F–4SF according to the diagram as follows:
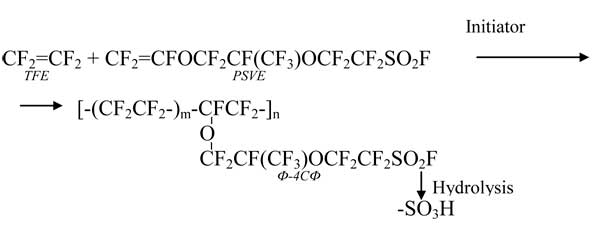
Following the film extrusion the copolymer sulfonylfluoride groups are hydrolysed to form sulfoacidic ones.
After 1960ies this well-known co-polymer is widely used in the manufacture of proton-conducting membranes (Nafion, В«Du PontВ», USA; MF-4SK, Russia [1-3]).
A number of patents disclosed the methods of fluoroplast-4SF manufacture by the radical co-polymerisation in solution [4-7], in mass (without solvent) [7, 8], or by water-emulsion methods [9-17].
Earlier we have studied some aspects of TFE/PSVE co-polymerisation kinetics [18, 19]. We determined the co-polymerisation constants for TFE (r1=9.0) and PSVE (r2=0.04), Alfrey-Price parameters for PSVE: Q=0.02 and ГЄ=2.23, and the order of reaction by initiator n=0.7В±0.1.
The objective of this our study is to investigate both temperature and monomer concentration effects on the rate of TFE and PSVE co-polymerisation.
Experimental
Co-polymerisation was conducted in a steel 200ml reactor, equipped with a jacket and a frame stirrer (n=300 rpm). The reactor was ultra-thermostatted with accuracy В±0.1 В°C. CFC113 (1,1,2-trifluoro-1,2,2-trichloroethane) was used for solvent, and bis(perfluorocyclohexanoyl)peroxide (DAPc) was used for initiator. Tetrafluoroethylene was preliminary cleaned from inhibitor (triethylamine) in an adsorber charged with activated carbon. During the experiment the reactor pressure (and therefore, TFE concentration in liquid) was maintained constant by continuous TFE feeding from a calibrated buffer cylinder. TFE consumption was estimated by the pressure in the buffer cylinder. The polymer was washed with chloroform, and water, and then dried under vacuum at 80В°C to constant weight.
The copolymer composition was determined by the number of sulfonylfluoride groups in its IR-spectra [20], elemental analysis of sulfur content [21], and by the titration of sulfoacidic groups in hydrolysed copolymer.
The copolymer molecular mass was estimated by its melt flow index MFI with the help of a constant-pressure capillary viscosimeter (load 2.16 kg, filier diameter 2.095 mm, temperature 220Г·270В°C).
All tests were conducted at initial equimolar concentration ratio of the monomers TFE:PSVE = 1:1, and the initiator original concentration 6.7В·10-4 mole/l.
The results are shown in Tables 1, 2 and Figure 1.
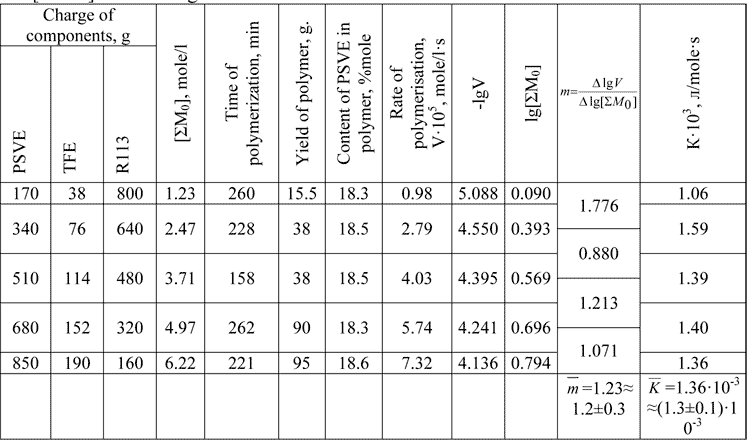
Table 1. The dependence of TFE/PSVE co-polymerisation rate on the monomer concentrations in CFC -113 environment at 35В°C, mole ratio TFE:PSVE=1:1, and [DAPc] initiator original concentration 6.7В·10-4 mole/l.
The co-polymerisation was depicted by a simplified equation as follows:
V=K·[DAPc]n·[∑M0]m, mole/l·s    (1)
here: Рљ is effective rate constant of the co-polymerisation process, mole/l;
[DAPc] is the
concentration of initiator, mole/l;
[∑M0] is combined initial concentration of
the monomers, mole/l;
n is the reaction order by initiator;
m is the reaction order by
the combined concentration of the monomers.
The reaction order by monomers was determined for each pair of tests according to the equation (2), derived according to the diagram as follows:
V1=K·[DAPc]0n·[∑M0]1m;
V2=K·[DAPc]0n·[∑M0]2m;
lgV1=lgK+nlg[DAPc]0+mlg[∑M0]1;
lgV2=lgK+nlg[DAPc]0+mlg[∑M0]2;
lgV1-lgV2=m(lg[∑M0]1-lg[∑M0]2), from where
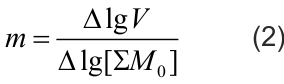
The effective rate constant of co-polymerisation was calculated by the equation (3):
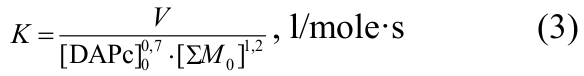
Reasoning from the experimental (Table 1) and published results [19] for TFE /PSVE co-polymerisation in CFC113 at 35В°C the equation (1) is:
V=(1.3±0.1)·10-3·[DAPc]0.7±0.1·[∑M0]1.2±0.3, mole/l·s     (4)
Our results for temperature dependence of the rate of TFE /PSVE co-polymerisation are shown in Table 2 and Figure 1.
Table 2. Temperature dependence of TFE /PSVE co-polymerisation rate in CFC113 environment at TFE:PSVE=1:1 mole ratio and the initiator initial concentration [DAPc]= 6.7В·10-4 mole/l.
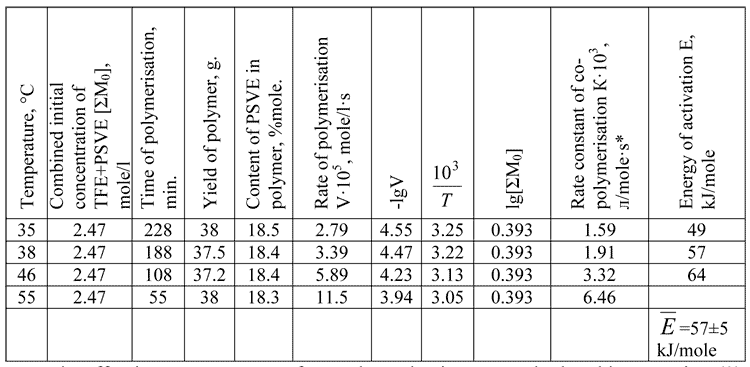
Note*: Effective rate constant of co-polymerisation was calculated by equation (3).
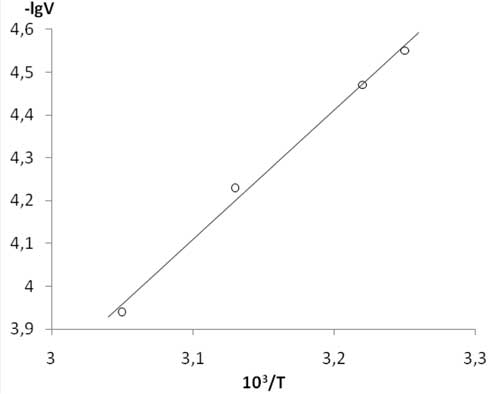
Figure 1. Temperature dependence of TFE/PSVE co-polymerisation rate in Arrhenius coordinates.
Energy of activation of TFE / PSVE co-polymerisation within temperature range 35-55 В°C is 57В±5 kJ/mole (Table 2, Figure 1).
Conclusions
- The kinetic parameters are determined experimentally for the radical co-polymerisation of TFE /PSVE monomers, used in the manufacture of perfluorinated ion-exchange membranes.
- he obtained values of the co-polymerisation rate constants, activation energies, reaction orders by combined monomer concentrations and by initiator concentrations are typical for the reactions of radical co-polymerisation of perfluorinated alkylvinyl ethers in solutions [8]; the process is heterogenous because F-4SF co-polymer thus formed is swelled in the reaction medium but not dissolved in it.
The study was conducted within the framework of State Contracts # 12411.1007499.09.174 of 21.09. 2012 and # 11.519.11.6024 of 25.10.2011.
References
2. Yamabe M. Novoe v tekhnologii soedinenij ftora. M.: Mir, 1984. s. 336-354.
3. Kirsh Yu. E., Smirnov S. A., Popkov Yu. M., Timashchev S. F.// Uspekhi khimii. 1990. t. 59. №6. s. 970-994.
4. Pat. 3282875 US, US Cl 524/795. Fluorocarbon vinyl ether polymers.
5. Pat. 3528954 US, US Cl 526/206. Process for homopolymerization of tetrafluoroethylene and copolymerization of same with fluoro co-monomers in the solvent 1,1,2-trichloro-1,2,2-trifluoroethane.
6. Pat. 5281680 US, US Cl 526/243. Polymerization of fluorinated copolymers
7. Pat. 4116888 US, US Cl 521/28. Process for producing fluorinated copolymer having ion-exchange groups.
8. Pat. 4349650 US, US Cl 526/243. Polyfluoroallyloxy compounds, their preparation and copolymers therefrom.
9. Pat. 62-288614 JP, MKI C 08 J 214/26. Sposob polucheniya perftorkarbonovogo sopolimera, soderzhashchego sul'fogruppy.
10. Pat. 62-288615 JP, MKI C 08 J 214/26. Sposob polucheniya perftorkarbonovogo sopolimera, soderzhashchego sul'fogruppy.
11. Pat. 62-288616 JP, MKI C 08 J 214/26. Sposob polucheniya perftorkarbonovogo sopolimera, soderzhashchego sul'fogruppy.
12. Pat. 62-288617 JP, MKI C 08 F 214/26. Sposob polucheniya perftoruglerodnogo polimera, soderzhashchego sul'fokislotnye gruppy.
13. Pat. 3282875 US, US Cl 260-29.6. Fluorocarbon vinyl ether polymers.
14. Pat. 4329435 US, US Cl 521/38. Novel fluorinated copolymer with tridihidrofuorosulfonul fluoride pendant groups and preparation thereof.
15. Pat. 4330654 US, US Cl 526/243. Novel polumers having acid functionality.
16. Pat. 4536352 US, US Cl 260/543F.Perfluoro vinyl ethers.
17. Pat. 2267498 RF, MPK S 08 F 214/26. Linejnyj statisticheskij terpolimer tetraftorehtilena s funkcional'nymi perftorirovannymi somonomerami i sposob ego polucheniya.
18. Zhurnal Prikladnoj Khimii.- 2009, t.82, №1.- s.113-116.
19. Sbornik «Ftorsoderzhashchie membrano-kataliticheskie polimernye materialy i sistemy».- 2010.- SPb.- s.69-72.
20. Zhurnal Prikladnoj Khimii.- 2009. T.82. №1. s.2043-2047.
21. Zhurnal Analiticheskoj Khimii.- 1976. T.31. №6. s. 1212-1214.
Recommended for publication by V. Kornilov
Fluorine Notes, 2013, 88, 5-6
