Received: November , 2012
Fluorine Notes, 2013, 88, 1-2
The impact of fluorinated urethanes on the structure and properties of polyethyleneterephthalate
. V. Kudashev*, U.R.Urmantzev**, P.A.Matizin**, V. N. Arisova*
*Russia, Volgograd State Technical University, 400131, Volgograd, Lenina ave. 28
** JSC "POLYEF", Russia, Bashkortostan, 453434, Blagoveschensk
e-mail: kudashev-sv@yandex.ru
Abstract: The surface modification of polyethyleneterephthalate granulate is conducted with fluorinated prepolymers containing urethane groups. It was found that the modification involves predominantly the surface layer, 12-28 Вµm. The fluorinated urethanes introduction was shown to produce stabilizing effect on such polyethyleneterephthalate characteristics as the uptrend in its hydrolytic stability and thermal-oxidative stability.
Keywords: modification, polyethyleneterephthalate, polyfluorinated substances, urethanes.
INTRODUCTION
The modification of polymers in order to prepare some materials with novel or improved properties attracts permanent interest of researchers because original materials very often do not possess the set of properties and characteristics, required for their some or other practical application.В The use of polyethyleneterephthalate (PETPH) in the manufacture of materials for wide range of application requires some universal methods for their stabilization, not yet achieved with the help of available organic or mineral modifiers [1, 2]. Thereto poly- and perfluorinated substances are of unquestionable interest because they provide essential improvement of a number of properties (thermo-, light-, wear- resistance, and hydrolytic stability) of hetero-chained polymers even when their content is small (10-3 Г· 5 % mass) [3, 5-10]. At the same time the use of fluorinated diols in the synthesis of modified PETPH results in their poor etherification by acids and hence hindering of goal-oriented full-scale manufacture of polyester with improved set of properties [4].
This study objective was to investigate the structure and peculiarities of PETPH-granulate surface modification by fluorinated urethanes having in mind the production of polyesters with improved hydrolytic and thermal-oxidative stability.
EXPERIMENT
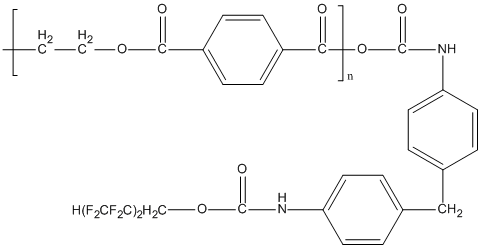
The polyester sample here applied was high-viscous (crystallized) or amorphous PETPH-granulate (granule average size 3x3 mm) supplied by JSC "Polyef" (Blagoveschensk, Rep.Bashkortostan) with the terminal carboxy group content 30 mmol/kg or 40 mmol/kg correspondingly (TU 2226-008-39989731-2009). In the polyester samples modification we used fluorinated urethanes (FU) that was a blend of prepolymers (mono- and diurethane, 1,4-disubstituted uretidindion, 1,3,5-trisubstituted isocyanurate), produced simultaneously by the reaction of 4,4'-diphenylmethanediisocyanate with 1,1,5-trihydroperfluoropentanol-1 [5].
Within 0.5-3.0% mass FU concentration range the surface modification of PETPH-granules was carried out by solvent-based method with the help of chlorobenzene (AR grade).
Example of modification technique. A glass flask was charged with 100g of PETPH granulate, 2g of FU, and 300ml of chlorobenzene. The flask was thermostated at 120В°C during 4 hours, and then solvent was distillled. The resulting surface-modified PETPH granulate was dried at 100В°C under vacuum.
Surface-modified PETPH-granules were studied by impulse solid-phase NMR at 300.13 MHz and 27В°C (Bruker AVANCE-300 spectrometer), X-ray reflecion-diffractometery (DRON-3 automatic diffractometer, CuРљО± radiation (О» = 1.5418 Г…)), IR-Fourier spectrometery (Nicolet-6700 spectrometer), air-thermogravimetry (TGM) (Q-1000 spectrometer-derivatograph, F.Paulik, J.Paulik, L.Erdey system (MOM, Hungary)), differential scanning calorimetry (DSC, Netzsch DSC 204 F1 Phoenix, and Mettler Toledo DSC 822e calorimeters), and atomic-force scanning probe microscopy (SPM, Solver PRO microscope with silicon probe, 40N/m rigidity and 10nm radius of needle curvature). For TGM and DSC curves recording the sample mass was 16-20 mg, the temperature growth rate was 10В°C per minute. The experimental diffractograms were processed with the help of Fit2D software.
The characteristic viscosity was measured with the help of В«UbbelohdeВ» glass capillary viscosimeter (1C type by ISO 3105) by dissolving the polymer sample in a solvent blend (phenol : tetrachloroethane (60:40 by mass)). Colour index values L* (index of lightness that characterizes white-black brightness) and b* (index of colour that characterizes blue-yellow brightness nuance) were determined on the basis of differential colorimetry according to CIE International Colorimetry System.
The detection of carboxyl group part by mole based on sodium hydroxide titration of PETPH sample dissolved in o-cresol : chloroform (70:30 by mass) solvent blend, followed by reverse titration with hydrochloric acid.
The estimation of PETPH humidity was done by the method of coulometry based on the determination of the amount of water evaporated in a drying oven from the polyester granule surface and carried by nitrogen flow into Fisher automatic titration station.
DISCUSSION OF RESULTS
The introduction of 2% FU into polyester matrix shifts its destruction temperature (T5% Г· T40%) to higher temperature range (Table 1). However, we failed to increase considerably the destruction temperature using the solution method for FU introduction. It might be due to relatively uneven distribution of the additive within PETPH surface layer, or some uptrend in the submolecular structure imperfection in the process of the solution removing that resulted in certain loosening of the polymer inter-structure sections.
Table 1. Thermal-oxidative stability of original and modified PETPH.
|
Material |
Temperature of corresponding mass loss, В°C |
|||||||
|
Tstart |
T5% |
T10% |
T15% |
T20% |
T30% |
T40% |
T50% |
|
|
Crystal PETPH |
||||||||
|
PETPH |
282 |
368 |
382 |
387 |
392 |
394 |
400 |
487 |
|
PETPH + 0.5 % FU |
284 |
370 |
388 |
390 |
393 |
397 |
406 |
420 |
|
PETPH + 1.0 % FU |
281 |
378 |
391 |
394 |
395 |
399 |
414 |
427 |
|
PETPH + 1.5 % FU |
283 |
385 |
397 |
395 |
400 |
418 |
420 |
448 |
|
PETPH + 2.0 % FU |
284 |
396 |
407 |
416 |
419 |
421 |
425 |
465 |
|
PETPH + 3.0 % FU |
275 |
365 |
383 |
385 |
389 |
395 |
425 |
453 |
|
Amorphous PETPH |
||||||||
|
PETPH |
248 |
342 |
352 |
355 |
358 |
375 |
386 |
463 |
|
PETPH + 2.0 % FU |
257 |
376 |
388 |
395 |
416 |
419 |
608 |
612 |
The analysis of fragile cross-chips in PETPH-granules by the method of AFM-microscopy evidenced that modification had involved mostly the surface layer, sized 12-18 mcm (crystal polyester) or 22-28 mcm (amorphous polyester). In its turn, if the FU content increases up to 3 % it makes much more difficult to provide even distribution of the additive within the polymer sample and entails negative effects on the polyester thermal-oxidative stability as well (Fig. 1).
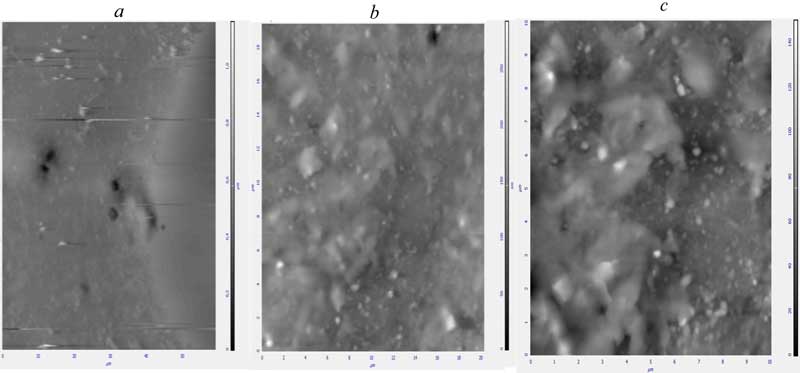
Fig. 1. Microphotography of crystal PETPH-granule fragile chips. a – PETPH + 1 % FU; b – PETPH + 2 % FU; c – PETPH + 3 % FU.
For better understanding of the sequence of alterations in crystal and amorphous PETPH due to heating or cooling one may use the DSC method (Fig. 2). Thus, the mobility of structure units in the macromolecular chain is represented by corresponding temperature transition coefficients (Table 2) [1, 2]:
- О±-transition is related to the loss in the mobility of the macromolecule structure units due to the start of crystallization;
- ОІ-transition characterizes the enhancement of rotation movement of the methylene groups belonging to glycol residue and aromatic nucleus;
- Оі-transition depicts the cessation of rotation movement of methylene groups in gauche- and trans-conformations in amorphous phase of the said polyester; this low-temperature transition has effect both on barrier properties and gas-permeability of the polymer.
Table 2. Thermal transitions and degrees of crystallinity in original and modified PETPH
|
Material |
Degree of crystallinity, % |
Thermal transitions, В°C |
||
|
О±-transition |
ОІ-transition |
Оі-transition |
||
|
Crystal PETPH |
||||
|
PETPH |
48 |
168 |
78.4 |
-47 |
|
PETPH + 2.0 % FU |
54 |
192 |
66.2 |
-58 |
|
Amorphous PETPH |
||||
|
PETPH |
30 |
128.9 |
75.4 |
-50 |
|
PETPH + 2.0 % FU |
41 |
120.1 |
65.6 |
-48 |
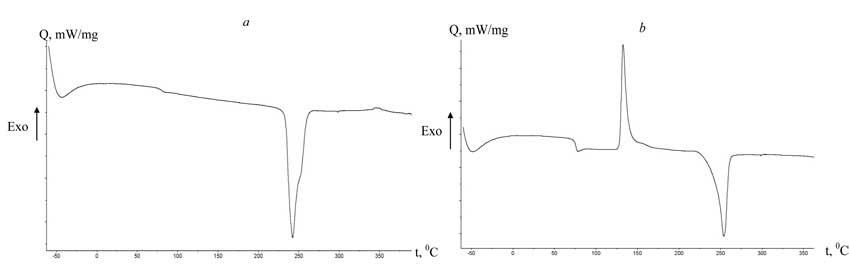
Fig. 2. Curves of DSC PETPH-images. Q – heat flow; t – temperature. a – crystal PETPH; b – amorphous PETPH.
The analysis of data in table 2 implies the growth of О±-transition temperature by 24В°C for crystal PETPH-samples and by 8.8В°C for amorphous polymer sample, containing 2% FU, that are evidence for the association ~C=O (PET) в€™в€™в€™ H-N~ (FU), and for the intermolecular interactions between fluorine atoms belonging to the perfluorocarbon chain of the modifier and methylene groups of the glycole residue, resulting in the stabilization of submolecular polyester structure. The reduction in the value of Оі-transition by 11В°C evidences for that fact that FU may perform as nucleation centers and branching agents of the macromolecular chain [1, 2].
The study of modified PETPH structure by the method of impulse NMR in solid phase allowed to conclude about its good conformity with DSC data. NMR 1H spectra involve a wide О -shaped line with width at semi-height О”ОЅ = 39000 Hz, and a narrow central line with width at semi-height О”ОЅ = 2700 Hz, and chemical shift of 6.5 ppm (Fig. 3). The introduction of 2 % mass FU into the polymer sample hinders the rotation movement of its structural fragments within its modified sections:
and, therefore, the general freedom of the macromolecular chain, that is related to cooperative associational interactions between the modifier and the polymer and represented by weakening of the said central narrow line (integral intensity of the total signal fells from 0.28 % to 0.22 %), and by some increase in the О -shaped line half-width. The result is particularly valuable when it concerns the manufacture of polyester materials with small coefficients of gas diffusion through the matrix.
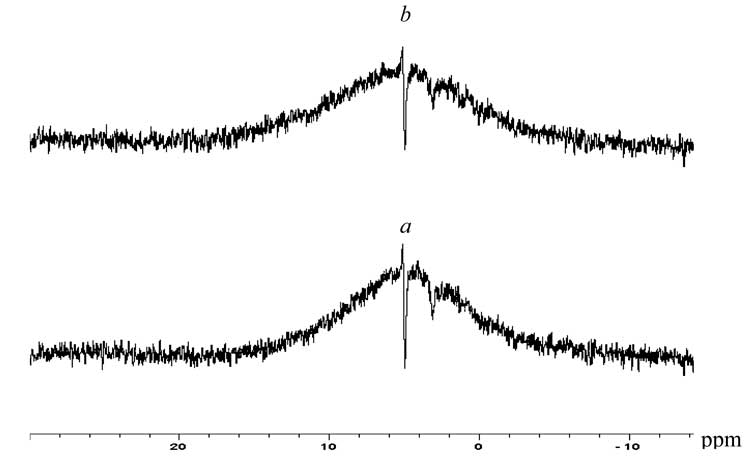
Fig. 3. Impulse solid-phase NMR 1H spectra of original (a) and modified (b) crystal PETPH
The alterations in PETPH X-ray spectrograms due to the impact of 2 %mass FU were analyzed. The diffractograms of the studied samples are all of the same type: there is predomination of diffraction peaks 010, 110, and 100, superimposed with amorphous halo (Table 3). Two more peaks with considerably lower intensities are observed at dissipation angles of 42.6В° and 55.9В°. In conformity with the X-ray study results obtained by a number of researchers [11-13], all ribs of PETPH elemental unit differ in their length and none of the angles is straight (90В°). Therefore, the space latitude is triclinic (a = 0.456 nm, b = 0.595 nm, c = 1.075 nm; О± = 98.5В°, ОІ = 118В°; Оі = 112В°). Taking into account those crystallographic data the indices 105 and 200 should be assigned to the observed reflexes correspondingly.
Table 3.Results of X-ray diffraction studies of PETPH-samples
The introduction of the modifier in amount of 2 % mass results in the alteration of the polyester structure evidenced by the re-distribution of its diffraction lines. Therefore, in spite of partial growth of the modified PETPH crystallinity degree, as it was shown by DSC method, there was some loss in the integral signal intensity, related to predominant В«concentrationВ» of links with trans-conformation, forming crystal phase, in surface layers, but not in the bulk polyester. The change in the location and intensity of the reflex 2Оё = 17.7В°, that corresponds to the reflection of 010 facet in PETPH crystal lattice, is indicative of relative density of those crystals, due to common macromolecule orientation processes, relaxation, and their nucleation centers formation.
The surface modification of amorphous PETPH with 2 % mass FU, results in the emerging of characteristic crystal phase reflexes with corresponding intensities. It is found that the barycenter of amorphous halo in PETPH experimental difractograms is revealed at ~19.8В° dissipation angle, while in that modified with 2 % mass FU it shifts to ~ 22.3В° (for amorphous modified PETPH ~ 21.0В°).
It is important that the introduction of 2 % FU into PETPH expands the vitrification temperature interval and shifts it to lower temperatures; some researchers assign this fact to the growth of polyester molecular mass due to its modification by its terminal groups (Table 4) [1, 2]. However, this apparent effect was not observed in the case of modified polymer melt temperature. The complex of results evidences of the formation of В«secondaryВ» sub-molecular structures within the surface zone of the modified polyester, that differ considerably in the buckle length of its macromolecular chains in crystals as to compare with original PETPH.
Table 4. Thermophysical properties of original and modified PETPH
The modification of PETPH-granule surface with FU contributes to its hydrolytic stability, in average, by 2 times (Table 5). Post-test microphotographies of the polyester granule surface after tsting in aggressive media typically have much less microspikes, cracks and slots, arising due to polymer destruction, because the common impact of the modifier perfluoroalkyl fragments and re-structuring of the macromolecular system due to the influence of introduced FU (Fig. 4).
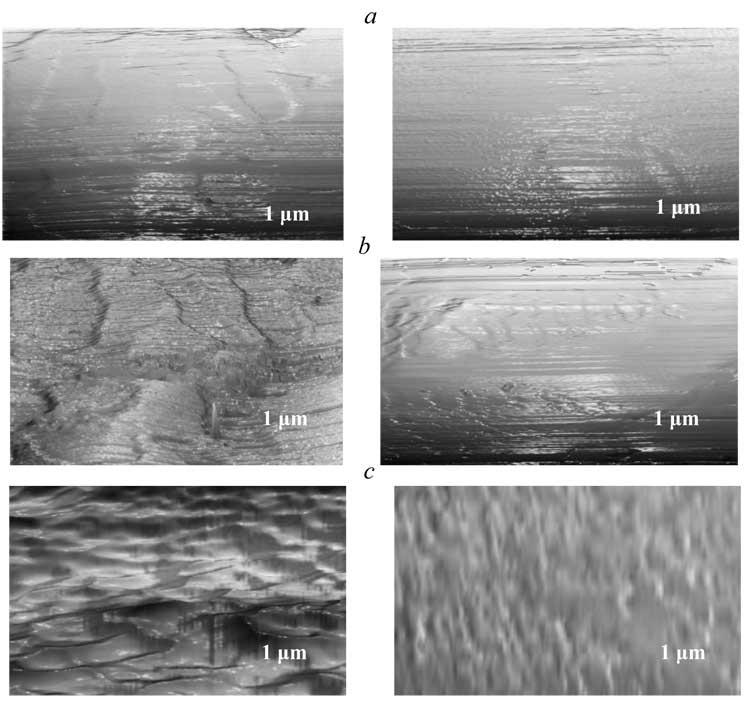
Fig.
4. Microphotography of crystal PETPH-granules surface after testing in aggressive media.
Left – original PETPH, right – PETPH + 2 % FU.
a – handling in water during 24 h;
b – boiling in 5 % hydrochloric acid at 120°C during 30 minutes;
c –
boiling in sodium hypoclorite solution at 80В°C during 30 minutes.
Table 5. Stability of original and modified PETPH in aggressive media
The polyester may contain both hydroxyl and carboxy terminal groups. At the production conditions strict control of those terminal group balance is very important both for the manufacture process regulation, and for PETPH processing. When so doing the PETPH terminal carboxy groups may catalyze hydrolysis at the polymer vitrification temperature or at higher temperatures thus entailing partial destruction of the macromolecule, particularly in the presence of humidity, followed by the formation of terephtalic acid and oligomeric white settling during extrusion or pressure die casting [1].
Aromatic isocyanates are able to react sufficiently actively with substances that contain В«activeВ» hydrogen [5]. The interaction of the polyester terminal carboxy groups with the modifier isocyanate groups may result in the formation of mixed anhydrides:
It is typical for the surface of modified samples of crystal or amorphous PETPH that carbonyl absorption bands are present in their spectra with frequencies that differ by 65.6 cm-1 , that allows to identify the anhydrate group in the structure of the prepared polyester (Fig. 5). It should be mentioned that the intensive absorption band typical for hydroxyl valent vibrations of carboxy group, weakens considerably and becomes wider, that points to the association of perfluorocarbon fragments with the proton of carboxy group. The absorption band form is influenced also by the vibrations of HN-group belonging to FU.
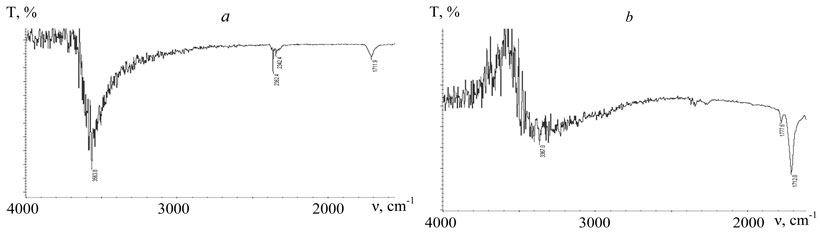
Fig. 5. IR-Fourier spectra of crystal PETPH-granules.
T –
transmission; ν – frequency.
a – original PETPH; b – PETPH + 2 % FU.
The presence of 1,3,5-trisubstituted isocyanurate and 1,3-disubstituted uretidindion in the FU blend may cause the cleavage of those cycles at temperature 300-350В°C entailing the additional generation of isocyanate groups able to interact with ~C(O)-NH~ fragments, resulting in cross-linked structures, that favor increase in the polyester thermostability [5].
Comparative analysis of the original and surface-modified polyester quality led to the conclusion that the introduction of 2% FU resulted in stable decrease in the content of acetaldehyde and humidity (Table 6). The loss in PETPH water content is particularly important because it may cause the destruction of polyester [1, 2].
Table 6. Quality indices for original and modified crystal PETPH
Therefore, we studied the impact of fluorinated urethanes on structural-morphological characteristics and properties of polyethyleneterephthalate granulate, that allows to increase the polyester thermal-oxidative stability (predominantly within temperature range 365-425В°C) and its hydrolytic stability (2 times growth in average) due to their combined effect on the polymer sub-molecular structure that results in the modification of its surface layer sized 12-28 mcm, and increase in the degree of the polymer crystallinity.
References
2. Petuhov B. V. Poliehfirnye volokna. M.: Khimiya, 1976.
3. Kudashev S. V. Diss. kand. khim. nauk. Volgograd, 2011.
4. Ponomarenko V. A., Krukovskij S. P., Alybina A. YU. Ftorsoderzhashchie geterocepnye polimery. M.: Nauka, 1973.304 s.
5. Rahimova N. A., Kudashev S. V.// Izv. VolgGTU. Seriya "Khimiya i tekhnologiya ehlementoorganicheskih monomerov i polimernyh materialov ". Vyp. 8 : mezhvuz. sb. nauch. st. / VolgGTU. - Volgograd, 2011. № 2. s. 133.
6. Rahimova N. A., Kudashev S. V.// Zhurnal obshchej khimii. 2011. T. 81, vyp. 7. s. 1181.
7. Rahimova N. A., Kudashev S. V.// Zhurnal prikladnoj khimii. 2010. T. 83, vyp. 11. s. 1905.
8. Rahimova N. A., Kudashev S. V.// Izv. VolgGTU. Seriya "Khimiya i tekhnologiya ehlementoorganicheskih monomerov i polimernyh materialov ". Vyp. 7 : mezhvuz. sb. nauch. st. / VolgGTU. - Volgograd, 2010. № 2. s. 49.
9. Novakov I. A., Rahimova N. A., Nistratov A. V., Kudashev S. V., Gugina S. Yu.// Trenie i iznos. 2011. T. 32. № 4. s. 344.
10. Novakov I. A., Rahimova N. A., Nistratov A. V., Kudashev S. V., Gugina S. Yu.// Khimicheskaya promyshlennost' segodnya. 2012. № 3. s. 31.
11. Daubeny R. P., Bunn C. W., Brown C. W. // Proc. Roy. Soc. 1954, A., v. 226. p. 531.
12. Lindner W. L. // Polymer, 1973, v. 13, № 1. p. 9.
13. Razumova L. L., Rudakova T. E., Moiseev YU. V. // Vysokomolek. soed., 1975, A. T. 17, № 4. s. 861
Recommended for publication by Prof. Alexander I. Rakhimov
Fluorine Notes, 2013, 88, 1-2
