Received: February , 2013
Fluorine Notes, 2013, 87, 3-4
SYNTHESIS AND STUDY OF ANTIBACTERIAL ACTIVITY OF SOME FLUOROSUBSTITUTED ACRIDONES DERIVATIVES
T.N.Kudryavtseva a, K.V.Bogatyrev a, P.I.Sysoev a, Yar Zar Htun a , L.G. Klimova b
aKursk State University, 305000 Kursk, Radishcheva, 33
bKursk State Medical University, 305041 Kursk, K. Marksa, 3
e-mail: labOS.kgu@mail.ru
Abstract: By N-alkylation of 2- and 4-fluoroacridones were synthesized 2- and 4-fluorosubstituted acids. New derivatives of 2-fluoroacridonacetic acid were obtained, their antibacterial activity was investigated.
Keywords: fluorosubstituted acridones, kinetic parametres of reactions, acridoneacetic acids derivatives, antibacterial activity.
The different biologically-active derivatives of acridone are successfully used presently in medicine as antitumoral [1, 2], antifungal [3,4], antimicrobial [5], antiviral [6,7] and antiparasitogenic [8] agents. One of perspective directions is a synthesis of fluorosubstituted acridones derivatives, among them are already found some substances with effective antitumoral [9-13], antimalarian [14] and insecticide action [15].
By the cyclization of 2'- and 4'- of fluorodiphenylamine -2-carboxylic acids in polyphosphoric acid (PPA) with concentration of Р 2O5 80В±0,5 % 4- and 2- fluoroacridones were synthesized:
Scheme 1
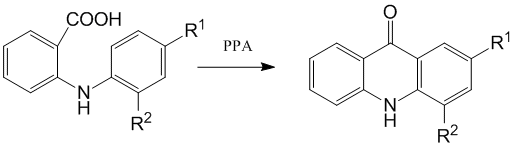
R1=H, R2=F; R1=F, R2=H
It was shown earlier that using of PPA for cyclization is more expediently than concentrated sulphuric acid, because it eliminates the side reaction of sulphonation of formed acridone and provides the quantitative yield of final product [16].
In continue of works about the study of diphenylamine-2-carboxylic acids (DPAA) kinetics of cyclization [16,17] by the method of TLC (with a densitometry) cyclization’s kinetic parameters of fluorodiphenylamine-2-carboxylic acids (FDPAA) in PPA with ratio FDPAA : PPA = 1 : 4 (on mass) were determinated. Kinetic studies were carried by the method of TLC with a densitometry. The obtained chromatograms analyzed on the videodensitometer "Denscan" at the wave-length 254 nm in the program "Sorbfil 1.8" [18].
After evaluation of chromatograms data the degree of conversion of initial FDPAA (О± FDPAA =C FDPAA /C FDPAA, 0) was determined, from the obtained kinetic curves their anamorphosises were built and the rate constants of reactions were calculated (Fig. 1, 2).
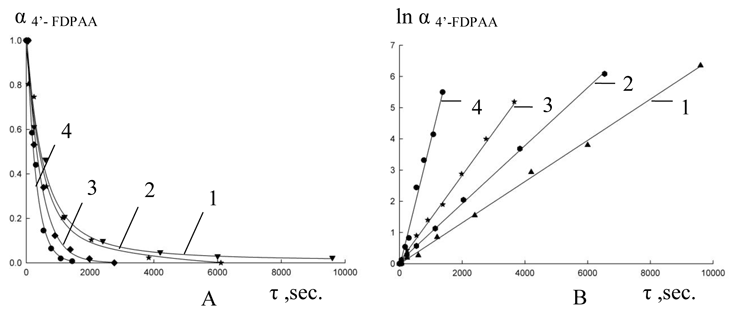
Fig 1. Kinetic curves of expense 4'-fluoro DPAA (A) at a cyclization in PPA and their anamorphosises (B) at different temperatures: 1-80 В°C, 2- 90 В°C, 3 - 100 В°C, 4-110В°C
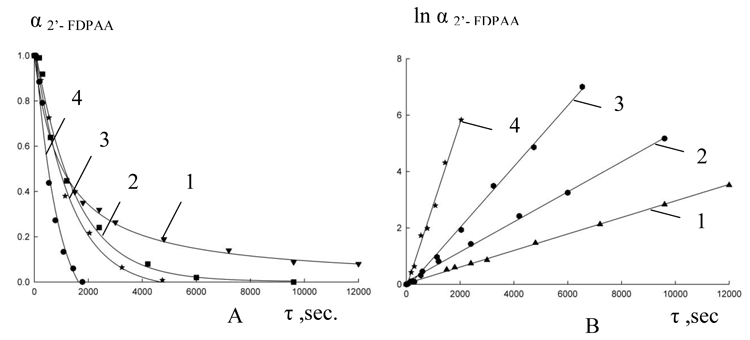
Fig. 2. Kinetic curves of expense 2'-fluoro DPAA (A) at cyclization in PPA and their anamorphosises (B) at different temperatures: 1-80 В°C, 2- 90 В°C, 3 - 100 В°C, 4-110В°C
From the data obtained we have calculated the activation energy for reaction of cyclization and to compare these results to the corresponding parameters for acridones with other substituents obtained before.
Table 1. Values of rate constants and activation energies for cyclization’s processes of substituted diphenylamine-2-carboxylic acids in PPA.
|
Initial FDPAA |
The rate constant, k Г— 10-4, sec-1, at T В°C |
Energies of activation, kJ/mol |
||||
|
70В°C |
80В°C |
90В°C |
100В°C |
110В°C |
||
|
Unsubstituted |
1,52В±0,07 |
4,13В±0,17 |
10,5В± 0,50 |
25,90В±1,11 |
- |
100,0В±4,4 [16] |
|
2’-CH3 |
0,70В± ,03 |
1,93В± 0,09 |
4,52В± 0,22 |
11,20В± 0,55 |
- |
93,0В±4,1 [16] |
|
4’-CH3 |
0,74В± ,04 |
3,19В± 0,13 |
5,51В± 0,23 |
15,40В± 0,63 |
- |
103,0В±5,1 [16] |
|
2'-Br |
6,60В± ,26 |
18,80В± 0,88 |
40,60В±2,01 |
119,80В± 4,80 |
– |
95,0В±4,2 [16] |
|
2’-F |
- |
2,99В± 0,14 |
5,85В± 0,25 |
10,64В± 0,51 |
24,53В± 1,10 |
77,5В±3,8 |
|
4’-F |
- |
6,89В±0,28 |
12,24В±0,49 |
16,77В± 0,83 |
28,92В± 1,36 |
54,0В± 2,6 |
As follows from the table 1, the values of rate constants of cyclization’s reactions of fluorosubstituted diphenylamine -2-carboxylic acids approached to the corresponding values of speed constants for methylsubstituted DPAA. Cyclization 2'-bromodiphenylamine-2-carboxylic acid is characterized by the higher values of reaction's constants of rate as compared to 2'-fluorodiphenylamine-2-carboxylic acid.
Thus introduction of fluorine atom to the molecule of diphenylamine -2-carboxylic acid results to the very considerable decline of activation energy of its cyclization process to corresponding acridone.
Synthesized fluoroacridones were alkylated by butyl chloroacetate with NaH in DMF.
Scheme 2
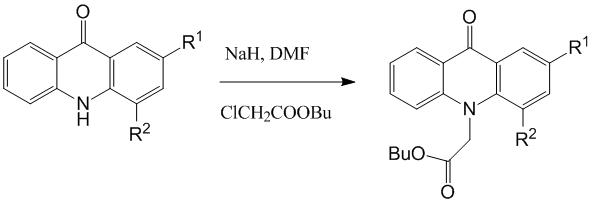
R1=H, R2=F; R1=F, R2=H
It was shown before that acridones with substituent in position 4 (CH3, Br, NO2, COOH) have been alkylated by butyl ester of chloroacetic acid in DMF extraordinarily difficultly and with very small yield, probably, in connection with steric difficulties. In case of 4-fluoroacridone we carried this reaction with 54 % yield. Presumably, it can be explained by the relatively small size of effective atom radius of fluorine.
2-Fluoroacridoneacetic acid butyl ester was synthesized with nearly quantitative yield, therefore, due to the higher availability of this compound as compared with ester of 4-fluoroacridoneacetic acid, namely it was used for the preparation of derivatives with potential biological activity.
2-(4-methyl-1,3-thiazole-5-yl)-ethyl-(2-fluoro-9-acridone-10-yl) acetate was synthesized by transesterification of 2-fluoroacridoneacetic acid butyl ester (2-F-AAA) in the medium of 4-methyl-5-(2-hydroxyethyl)-thiazole using sodium methylate as a catalyst (1a):
Scheme 3

Ability of thiazolyl ester 1a to form a water-soluble hydrochloride was used for purification of that product from the starting butyl ester.
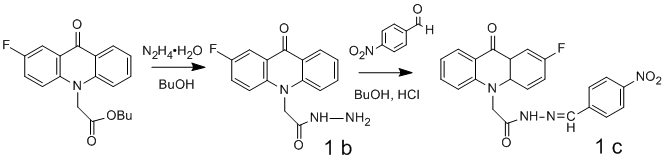
Hydrazide 2-F-AAA (1b) was obtained by hydrazinolysis of 2-F-AAA butyl ester. Arylidenehydrazide 2-F-AAA (1c) was prepared by condensation of hydrazide 2-F-AAA (1b) and 4-nitrobenzaldehyde. This reaction rapidly proceeds at room temperature with acid catalysis in the medium of butanol.
Scheme 4
Products 1a, 1b and 1c (solutions of various concentrations in DMSO) were tested for antimicrobial activity against the test strains of microorganisms.
The results of microbiological tests are shown in Table 2.
Compound 1a showed high activity against Candida albicans, comparable to standard drug of acridine series rivanol. Compound 1b and 1c were also highly active against gram-positive and gram-negative microorganisms. Furthermore arylidenehydrazide 1c surpassed rivanol to all studied test strains. Comparison of antibacterial activity of the obtained derivatives of 2-F-AAA with similar derivatives of unsubstituted acridoneacetic acid showed that the fluorinated substances 1a, 1b and 1c more effectively inhibited the growth of microorganisms than their unsubstituted analogues.
Table 1. Antibacterial activity of compounds 1a, 1b, 1c
|
Compound |
C, % |
E. coli (ATCC 25922) |
Ps. aeruginosa (ATCC 27853) |
Pr.vulgaris (ATCC 4636) |
S. aureus (ATCC 25923) |
B.subtilis (ATCC 6633) |
Candida albicans (NCTC2625) |
|
Zone of growth inhibition, mm |
|||||||
|
1a |
0,5 |
8,00В±0,62 |
10,00В±0,37 |
12,25В±0,87 |
14,25В±0,87 |
9,75В±0,82 |
14,50В±0,62 |
|
1,0 |
8,80В±0,71 |
10,05В±0,94 |
11,50В±0,32 |
14,00В±0,75 |
10,0В±0,37 |
13,50В±0,94 |
|
|
2,0 |
9,00В±0,75 |
13,50В±0,66 |
15,00В±1,09 |
14,50В±0,97 |
11,50В±0,32 |
15,00В±1,09 |
|
|
1b |
0,5 |
14,25В±0,57 |
9,50В±0,50 |
10,00В±0,37 |
12,00В±0,23 |
9,05В±0,37 |
14,25В±0,30 |
|
1,0 |
13,50В±0,66 |
10,00В±0,37 |
12,75В±0,38 |
15,00В±1,09 |
11,50В±0,32 |
17,00В±0,43 |
|
|
2,0 |
16,75В±0,38 |
14,75В±0,38 |
14,25В±0,57 |
18,75В±0,82 |
14,25В±0,57 |
19,25В±0,78 |
|
|
1c |
0,5 |
15,50В±0,66 |
14,25В±0,57 |
14,50В±0,50 |
17,25В±0,75 |
11,50В±0,32 |
18,75В±0,82 |
|
Rivanol |
1,0 |
12,75В±0,47 |
12,00В±1,14 |
12,50В±0,83 |
17,00В±1,02 |
14,05В±0,94 |
13,50В±0,56 |
|
2,0 |
14,50В±0,57 |
15,00В±0,93 |
15,00В±0,66 |
20,00В±0,97 |
15,00В±1,14 |
15,00В±0,96 |
|
Experimental
Purity of the starting materials and the synthesized compounds was confirmed by TLC (Type В«SorbfilВ», eluent - toluene: acetone: ethanol at volume ratio 10:3:2). The composition and structure of substances were confirmed by IR spectroscopy (FTIR spectrometer FSM 1201 Monitoring, KBr tablets), LCMS (system ACQUITY UPLC H-Class with UV/ mass detectors ACQUITY SQD Waters), UV spectroscopy (UV-spectrometer UV-1800 Shimadzu).
Cyclization FDPAA to the corresponding acridone was performed according to the described procedure [18], N-alkylation of acridone - according to the method [19] with some modifications (the reaction was carried at 110 В°C for 4 hours with momentary addition butyl chloroacetate).
2- Fluoroacridone. The yellow crystalline substance. Yield: 96 %. m.p.>340 В°C. Rf =0,62. Mass spectrum, m/z (Irel %): 214 [M+H]+ (100). IR spectrum (KBr) ОЅ, cm -1: 3458-2933 (N-H, C-H), 1634 (C=O), 1603-1482 (C-Car). UV-vis spectra (MeOH), О»max/nm (lg Оµ): 406 (0,506); 386 (0,502); 247 (2,19); 212 (0,998).
4- Fluoroacridone. The yellow crystalline substance. Yield: 96 %. m.p.=318 В±2 В°C. Rf =0,76. Mass spectrum, m/z (Irel %): 214 [M+H]+ (100). IR spectrum (KBr) ОЅ, cm -1: 3466-2952 (N-H, C-H), 1630 (C=O), 1603-1481 (C-Car). UV-vis spectra (MeOH), О»max/nm (lg Оµ): 395 (0,399); 377 (0,403); 251 (2,497); 215 (1,055).
2-Fluoroacridoneacetic acid butyl ester. Pale-yellow solid. Yield: 94 %. m. p. = 148 ±2 °C. Rf =0,84. Mass spectrum, m/z (Irel %): 328 [M+H]+ (100), 272 [C15H10FNO3+H]+ (36). IR spectrum (KBr) ν, cm -1: 2961 - 2876 (C—H); 1738 (C=O); 1620 (C=Oacridone), 1601, 1504, 1494, 1465 (C-C ar). UV-vis spectra (MeOH), λmax/nm (lg ε): 404 (0,396), 386 (0,340), 258 (1,455), 248 (1,652) .
4-Fluoroacridoneacetic acid butyl ester. Yellow solid. Yield: 54 %. m.p. = 142±2 °C. Rf =0,82. Mass spectrum, m/z (Irel %): 328 [M+H]+ (100), 272 [C15H10FNO3+H]+ (71), 226 [C14H10FNO – H]+ (12), 213 [C13H8FNO]+ (4). ). IR spectrum (KBr) ν, cm -1: 2958 - 2871 (C—H); 1752 (C=O); 1636 (C=Oacridone), 1602, 1501, 1460 (C-Car). UV-vis spectra (MeOH), λmax/nm (lg ε): 395 (0,807), 252 (2,605), 216 (1,674).
2-(4-Methyl-1,3-thiazol-5-yl)-ethyl-(2-fluoro-9-acridone-10-yl)acetate (1a). Mixture of 1 g (0,0031 mol) 2-fluoroacridoneacetic acid butyl ester, 6 g (0,042 mol) 4-methyl-5-(2-hydroxyethyl)thiazole and 20 Вµl NaOCH3 (20% in CH3OH) stirred at 120 В°C for 4 hours. The reaction mixture poured into water, the formed precipitate was washed with hot water and dissolved in 50 ml of a 2% solution of HCl.
Insoluble precipitate (the starting butyl ether) was filtered off, the filtrate was neutralized with sodium carbonate solution. The precipitate was washed with water, dried and recrystallized from butanol.
Pale-yellow crystalline solid. Yield: 77 %. m.p. = 144 ±2 °C. Rf =0,60. Mass spectrum, m/z (Irel %): 397 [M+H]+ (100), 272 [C15H10FNO3+H]+ (10). IR spectrum (KBr) ν, cm -1: 3067 - 2924 (C—H); 1746 (C=O); 1638 (C=Oacridone), 1601, 1491, 1467 (C-Car), 1414 (thiazole ring). UV-vis spectra (MeOH), λmax/nm (lg ε): 405 (0,314), 386 (0,279), 247 (1,478).
2- Fluoroacridoneacetic acid hydrazide (1b). To a mixture of 0.5 g (0.0015 mol) 2-fluoroacridoneacetic acid butyl ester and 5 ml of BuOH was added 0.25 ml of hydrazine monohydrate, the reaction mixture was refluxed with stirring for 2 hours. The formed precipitate was filtered off and recrystallized from DMF.
The yellow crystalline substance. Yield: 84 %. m.p. = 285±2 °C. Rf = 0,49. Mass spectrum, m/z (Irel %): 286 [M+H]+ (100), 269 [C15H10 FN2O2 + H]+ (15), 226 [C14H10FNO – H]+ (15), 213 [C13H8FNO]+ (100). IR spectrum (KBr), ν/cm–1: 3437, 3317 (NH—NH2); 3001, 2918 (C—H); 1672 (C=O); 1615 (C=Oacridone); 1599, 1580, 1497, 1466 (C-Car). UV-vis spectra (MeOH), λmax/nm (lg ε): 407 (0,115), 388 (0,096), 247 (0,576), 291 (0,833).
N-2-(2-fluoro-9-oxoacridin-10(9H)-yl)-N'-(4-nitrobenzylidene)acetohydrazide (1c). A mixture of 0.5 g (0.0017 mol) 2-fluoroacridoneacetic acid hydrazideand 0.3 g (0.0019 mol) 4-nitrobenzaldehyde in 10 ml of BuOH, acidified with a few drops of concentrated HCl, was hold at room temperature and stirring for 1 hour. The resulting product was filtered off and recrystallized from DMF.
The yellow crystalline substance. Yield: 85 %. m.p. = 296±2 °C. Rf =0,63. Mass spectrum, m/z (Irel %): 419 [M+2H]+ (100). IR spectrum (KBr), ν/cm–1: 3432 (NH); 2924, 2849 (C—H); 1691 (C=O); 1620 (C=Oacridone); 1599, 1524, 1466 (C-Car). UV-vis spectra (MeOH), λmax/nm (lg ε): 405 (0,142), 387 (0,147), 321 (0,448), 248 (0,633).
Determination of antimicrobial activity was performed according to procedure [20]. Glass petri dishes set on the tables with strictly horizontal surface, was poured molten agar previously inoculated test strains of microorganisms.
Suspension of test organisms for seeding on Petri dishes were prepared by the turbidity standard by 10 units. As the seed used daily culture. A suspension of each microorganism were plated on a Petri dish. Microbial burden was 1000000 microbial cells in 1 ml.
In determining of the antimicrobial activity of the test compounds were prepared their solutions with concentrations 0,5%, 1.0% and 2.0% and placed in the center of the cylinder (0.1 mL). Then the plates were incubated at (37 В± 1) В° C for 18-20 h. Standard solution of rivanol at the same concentrations was used as a reference sample.
The diameter of the growth inhibition zones of test organisms were measured by micro-ruler to within 1 mm.
References
- Tabarrini O., Cecchetti V., Fravolini A., Nocentini G., Barzi A., Sabatini S., Miao H., Sissi C. Design and synthesis of modified quinolones as antitumoral acridones // J. Med.Chem. 1999. V. 42.N 12. P. 2136-2144.
- Sathish N.K., GopKumar P., Rajendra Prasad V.V.S., Shanta Kumar S.M., Mayur Y.C. Synthesis, chemical characterization of novel 1,3-dimethyl acridones as cytotoxic agents, and their DNA-binding studies // Med.Chem. Res. 2010. V. 19.N 7. P. 674-689.
- Ahua K.M., Ioset J.R., Ransijn A., Mauel J., Mavi S., Hostettmann K. Antileishmanial and antifungal acridone derivatives from the roots of Thamnosma rhodesica // Phytochemistry.2004. V. 65.N 7. P. 963–968.
- Meepagala K.M., Schrader K.K., Wedge D.E., Duke S.O. Algicidal and antifungal compounds from the roots of Ruta graveolens and synthesis of their analogs // Phytochemistry. 2005. V. 66.N 22. P. 2689–2695.
- Salimon J., Salih N., Yousif E., Hameed A., Kreem A. Synthesis and pharmacological evaluation of 9(10H)-acridone bearing 1,3,4-oxadiazole derivatives as antimicrobial agents // Arab. J. Chem. 2010.V. 3.N 4. P. 205–210.
- Yamamoto N., Furukawa H., Ito Y., Yoshida S., Maeno K., Nishiyama Y. Anti-herpesvirus activity of citrusinine-I, a new acridone alkaloid, and related compounds // Antiviral Res. 1989.V. 12.N 1. P. 21–36.
- Fujiwara M., Okamoto M., Okamoto M., Watanabe M., Machida H., Shigeta S., Konno K., Yokota T., Baba M. Acridone derivatives are selective inhibitors of HIV-1 replication in chronically infected cells // Antiviral Res. 1999.V. 43.N 3. P. 189–199.
- Lacroix D., Prado S., Kamoga D., Kasenene J., Bodo B. Structure and in vitro antiparasitic activity of constituents of Citropsis articulata root bark // J. Nat.Prod. 2011. V. 74. N 10. P. 2286–2289.
- Rajendra Prasad V.V.S., Peters G.J., Lemos C., Kathmann I., Mayur Y.C. Cytotoxicity studies of some novel fluoro acridone derivatives against sensitive and resistant cancer cell lines and their mechanistic studies // Eur. J. Pharm.Sci. 2011. V. 43.N 4. P. 217–224.
- Fadeyi O.O., Adamson S.T., Myles E.L., Okoro C.O. Novel fluorinated acridone derivatives. Part 1: Synthesis and evaluation as potential anticancer agents // Bioorg. Med. Chem.Lett. 2008. V. 18.N 14. P. 4172–4176.
- Rajendra Prasad V.V.S., Honeywell R., Rao J.V., Sathish N.K., Shanta Kumar S.M., Mayur Y.C. Characteristic fragmentation behavior of some novel anti-calmodulin acridone derivatives studied by electrospray ionization tandem mass spectrometry // Int. J. Mass Spectrom.2007. V. 263.N 2–3. P. 148–151
- Mayur Y.C., Zaheeruddin, Peters G.J., Lemos C., Kathmann I., Rajendra Prasad V.V.S. Synthesis of 2-Fluoro N10-substituted acridones and their cytotoxicity studies in sensitive and resistant cancer cell lines and their DNA intercalation studies // Arch.Pharm. 2009. V. 342.N 11. P. 640 – 650.
- Dorner B., Kuntner C., Bankstahl J.P., Wanek T., Bankstahl M., Stanek J., Müllauer J., Bauer F., Mairinger S., Loscher W., Miller D.W., Chiba P., Muller M., Erker T., Langer O. Radiosynthesis and in vivo evaluation of 1-[18F]fluoroelacridar as a positron emission tomography tracer for P-glycoprotein and breast cancer resistance protein // Bioorg.Med. Chem. 2011. V.19.N 7. P. 2190-2198.
- Winter R.W., Kelly J.X., Smilkstein M.J., Dodean R., Bagby G.C., Rathbun R.K., Levin J.I., Hinrichs D., Riscoe M.K. Evaluation and lead optimization of anti-malarial acridones // Exp. Parasitol. 2006. V. 114.N 1. P. 47–56.
- US Pat. 4474774 (1984)
- Pelevin N.A., Lotorev D.S., Lapin A.V., Brylev M.I., Gurov M.Yu. / Materialy mezhdunar. nauch. konf. molodyh uchenyh, aspirantov i studentov «Perspektiva-2008». Nal'chik: KBGU. 2008. T. III. S. 214-218.
- Markovich Yu.D., Kudryavceva T.N., Brylev M.I., Markovich V.Yu., Koroleva I.A. Kineticheskie parametry reakcii vnutrimolekulyarnoj kondensacii difenilamin-2-karbonovyh kislot v usloviyah mikrovolnovogo izlucheniya // ZhOKh. 2012. T. 82.N 1. S. 152–155
- Markovich Yu.D., Pelevin N.A., Kudryavceva T.N., Lotorev D.S. Izuchenie kinetiki reakcij ciklizacii difenilamin-2-karbonovyh kislot s ispol'zovaniem tonkoslojnoj hromatografii s densitometriej // Zav. labor.Diagnostika materialov. 2008. N 4. S. 7-10.
- Szulc Z., Mlochowski J., Palus J. Synthesis of carbocyclic derivatives of 9 (10H)-acridinone, 9H-carbazole and 10H-phenothiazine 5,5-dioxide as potential immunomodulating agents // Journal f. prakt. Chemie. 1988. V. 330.N 6. P. 1023-1029.
- Gosudarstvennaya farmakopeya SSSR / Min-vo zdravoohraneniya SSSR. Izd.11. Vyp.2. Moskva, izd. “Medicina”. 1990..
Recommended for publication by Prof. A. Eleev
Fluorine Notes, 2013, 87, 3-4
