Received: December , 2012
Fluorine Notes, 2013, 86, 9-10
Peculiarities of the Reaction of Perfluoroallyl Fluorosulfate with Sodium Phenolate in monoglyme
E.V. Igumnovaa, T.V. Strelkovab, N.D. Kagramanovb, A.A. Tyutyunovb, V.F. Cherstkovb, S.R. Sterlinb
aВ«P&M-InvestВ», ul. Vavilova 28, 119991, Moscow, Russia
bA.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, V-334, GSP-1, 119991 Moscow, Russia
e-mail: sterlins@yandex.ru
Abstract: It has been found that the reaction of perfluoroallyl fluorosulfate with sodium phenolate in monoglyme medium results in the formation of 1,3-diphenoxytetrafluoropropene as the main reaction product. This version of the reaction, hitherto not described, is accompanied by interaction of perfluoroallyl fluorosulfate with the solvent, that confirmed by formation of methyl perfluoroallyl- and methoxyethyl perfluoroallyl ethers – the products of monoglyme perfluoroallylation.
Keywords: : perfluoroallyl fluorosulfate; 1,3-diphenoxytetrafluoropropene.
The study of the reaction of O-nucleophiles with perfluoroallylating agents have more than a semi centenary history [1]. The appearance of such available and effective perfluoroallylating agent as perfluoroallyl fluorosulfate (1) [2,3] gave an additional impulse to this direction of organofluorine chemistry: based on fluorosulfate (1) various perfluoroallyl ethers have been synthesized [4], including the compounds promising from viewpoint of their industrial application [5].
Earlier we have found that among the products obtained by the reactions of perfluoroallyl- and perfluorobenzyl fluorosulfates with alkali halogenides in diglyme there were trace amounts of methyl perfluoroallyl- or methyl perfluorobenzyl ethers (according to CMS-data) (unpublished results).
The assumption that these ethers were formed by virtue of degradation of the corresponding oxonium ions – the products of electrophilic alkylation of diglyme by fluorosulfate 1 or perfluorobenzyl fluorosulfate – demanded experimental verification. As a subject of study we chose the reaction of fluorosulfate 1 with sodium phenolate, described in details earlier [4].
We’ve found that under the conditions of preparing phenylperfluoroallyl ether (2) (the addition of perfluoroallyl fluorosulfate 1 to a solution of equimolecular quantity of sodium phenolate in THF; yield of ether 2 87% [4]), but substituting monoglyme for THF as a solvent 1,3-diphenoxytetrafluoropropene (3) appeared to be the main reaction product. Up to our knowledge this version of the reaction of fluorosulfate 1 with O-nucleophiles hasn’t been described hitherto. At the same time the appearance of methyl perfluoroallyl ether (4) and methoxyethyl perfluoroallyl ether (5) among the reaction products (mol. ratio 4:5 ~ 1:1, totally 16 mol.% from 1, taken in the reaction), which formation is described by the scheme with participation of oxonium ion (6), confirms the above mentioned assumption comprising electrophilic alkylation of hydrocarbon ethers by perfluoroallyl fluorosulfate 1:
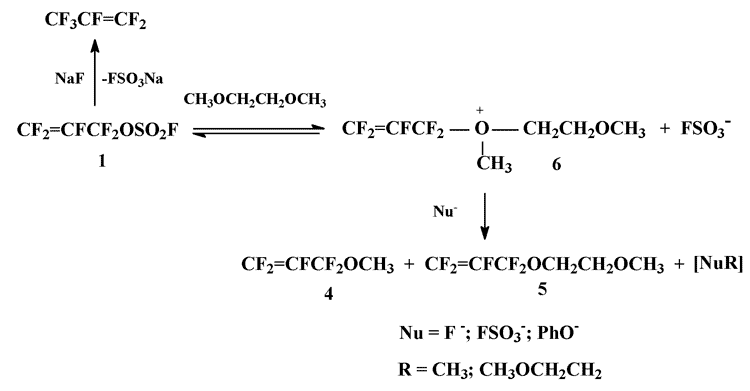
Scheme 1
The reaction is accompanied by evolution of gas, most probably hexafluoropropene, formed in the course of fluorosulfate 1 degradation under the action of NaF generated in situ.
Diphenoxypropen 3 is stable at ~25°C, but undergoes partial hydrolysis during distillation, that leads to the formation of a mixture of compounds 3,7-10 (mol. ratio 3:7:8:9:10 = 20:2:1:1:3), that can’t be separated by fractionation.
The sequence of transformations, leading to the compounds 7-10 is evident: the hydrolysis of diphenoxypropene 3 gives difluoroacrylate 8 that in its turn adds to 3 with the formation of 1,3-diphenoxy-2-hydroperfluoropropane 7. Two-stage hydrolysis of the latter results in formation of phenyl propionate 9 and diphenyl malonate 10.
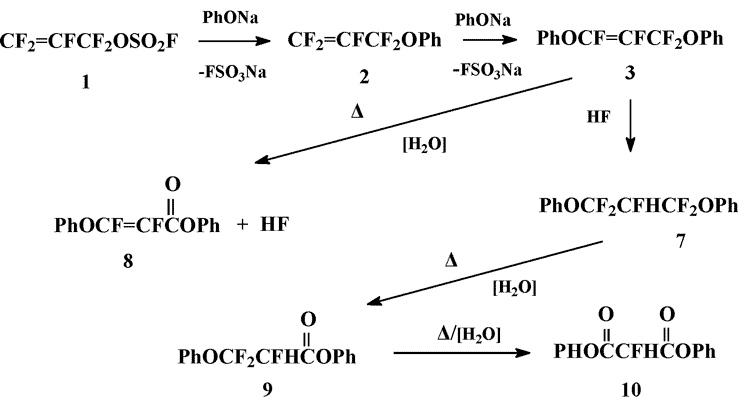
Scheme 2
The attempts to achieve deeper hydrolysis of the mixture obtained led to unexpected results. Thus, its short-term treatment with 40% aq. KOH (70-90В°C/8-10 min) afforded a mixture, which contained diphenoxypropene 3 and diphenyl malonate only (molar ratio 3:10 = 9:1).
In the presence of SiO2 the relative content of acrylate 8 begins to increase at ~25°C; when the reaction mixture is heated up to140-145°C vigorous exothermic reaction takes place, which results in the transformation of diphenoxypropene 3 - the dominant component – in to a mixture of difluoroacrylate 8, phenyl propionate 9 and diphenyl malonate 10 (molar ratio 7:8:9:10 = 1:3.8:1:1.7) (according to 19F NMR-data). Further heating (up to 170-175°C) leads to the growth of the relative content of diphenyl malonate 10, but under these conditions tarring of the reaction mixture is observed.
From the results obtained it follows, that perfluoroallyl fluorosulfate 1 is capable to alkylate hydrocarbon ethers even in the absence of electrophilic catalysis with the consequent formation of alkyl perfluoroallyl ethers. In connection with this finding we suggest an alternative route of preparing phenyl perfluoroallyl ether 2 by reaction of perfluoroallyl fluorosulfate 1 with triethylammonium phenolate in hexane. The reaction proceeds smoothly and affords ether 2 in 65% yield.

Scheme 3
Experimental
19F NMR spectra were recorded using a Bruker AVANCE-300 spectrometer at 282 MHz with D2O as an external standard. Chemical shifts in 19F spectra are given in ppm vs. CFCl3. Mass-spectra were recorded using a Finnigan Polaris Q (Trace GC ultra) mass-specrometer.
Perfluoroallyl fluorosulfate was prepared by method [2].
The reaction of perfluoroallyl fluorosulfate with sodium phenolate
The solution of 76 g (0,81 mole) PhOH in 250 ml of monoglyme was gradually (for 1 h) added to a mixture of 34 g of 60% suspension NaH in mineral oil (0,81mole) and 230 ml of monoglyme at 4-5В°C. Then 186 g (0,81mole) of fluorosulfate 1 was gradually (for 1.3 h) added to the solution obtained under stirring, sustaining the temperature of the reaction mixture at 15-18В°C (slightly exothermic reaction, gas evolution was observed), the mixture was stirred for 6 h, stayed overnight, the part of the solvent (220 ml) was distilled off at 35 Torr. The distillate obtained contained 68.2 mmol of methyl perfluoroallyl ether 4 and 60 mmol of methoxyethyl perfluoroallyl ether 5 (according to 19F NMR data, internal standard BrCF2CF2Br). The residue of monoglyme was distilled off into a trap (-78В°C) at 2-3 Torr; the distillate in the trap contained trace amounts of phenyl perfluoroallyl ether 2 (19F NMR data).
The residue was solved in CHCl3, the precipitate was filtered out and filtrate was evaporated under reduces pressure (2-3 Torr), the residue was distilled to give 74 g of a fraction boiling at 110-165В°C/1Torr, which contained compounds 3,7-10 (molar ratio 3:7:8:9:10: = 20:2:1:1:3 (19F РЇРњР ).
Table 1. 19F NMR spectra of compounds 3,7-10.

Mass-spectrum 4 (m/z, reference): 162 [M]+; 143 [M-F]+ (100%); 131 [C3F5]+; 128 [C3F4O]+; 112 [C3F4]+; 109 [C3F3O]+; 100 [C2F4]+; 93 [C3F3]+; 81 [C2F3]+; 78 [C2F2O]+; 69 [CF3]+; 47 [CFO]+; 31 [CF]+.
Mass-spectrum 5 (m/z, reference): 206 [M]+; 175 [M-CH3O]+; 131 [C3F5]+ (100%); 112 [C3F4]+; 100 [C2F4]+; 93 [C3F3]+; 74 [C3H3FO]+; 69 [CF3]+; 59 [C2FO]+; 47 [CFO]+; 45 [C2H5O]+; 43 [C2F]+; 31 [CF]+; 29 [CHO]+; 27 [C2H3]+.
Mass-spectrum 3 (m/z, reference): 298 [M]+; 279 [M-HF]+; 205 [M-PhO]+ (100%); 185 [C9H4F3O]+; 177 [C8H5F4]+; 139 [C8H5F2]+; 127 [C7H5F2]+; 109 [C3F3O]+; 77 [C6H5]+; 65 [C5H5]+.
Mass-spectrum 7 (m/z, reference): 318 [M]+; 225 [M-PhO]+; 205 [M-PhO-HF]+; 177 [C8H5F4]+; 141 [C7H3F2O]+; 127 [C7H5F2]+; 94 [C6H6O]+ (100%); 77 [C6H5]+; 66 [CF2O]+; 51 [CHF2]+.
Mass-spectrum 8 (m/z, reference): 277 [M+H]+; 259 [C15H9F2O2]+; 232 [M-CO2]+; 212 [C14H9FO]+; 183 [C9H5F2O2]+ (100%); 155 [C8H5F2O]+; 136 [C8H5FO]+; 127 [C7H5F2]+; 107 [C7H4F]+; 87 [C7H3]+; 77 [C6H5]+; 51 [CHF2]+.
Mass-spectrum 9 (m/z, reference): 296 [M]+; 277 [M-F]+; 257 [M-HF2]+; 225 [C11H7F2O3]+; 212 [C14H9FO]+; 203 [M-PhO]+; 183 [C9H5F2O2]+; 155 [C8H5F2O]+; 141 [C7H3F2O]+; 127 [C7H5F2]+; 109 [C7H6F]+; 94 [C6H6O]+ (100%); 77 [C6H5]+; 66 [CF2O]+; 51 [CHF2]+.
Mass-spectrum 10 (m/z, reference): 274 [M]+; 257 [C15H10FO3]+; 246 [M-CO2]+; 197 [C9H6FO4]+; 181 [M-PhO]+; 169 [C8H6FO3]+; 152 [C8H5FO2]+; 141 [C7H6FO2]+; 125 [C7H6FO]+; 105 [C7H5O]+; 94 [C6H6O]+ (100%); 89 [C7H5]+; 77 [C6H5]+; 66 [CF2O]+; 51 [CHF2]+
The reaction of perfluoroallyl fluorosulfate with triethylammonium phenolate
A solution of triethylammonium phenolate [from 73.6 g (0.78mole) of PhOH and 79 g (0.78mole) Et3N] in 550 ml of hexane was gradually added to a solution of 180 g (0.78mole) of fluorosulfate 1 at 10-15В°C, the reaction mixture was stirred at ~25В°C/3 h, then two-phase mixture was cooled in a bath (-78В°C). When the low layer solidified the upper layer was decanted, the solvent was evaporated under reduced pressure and the residue was distilled to give 116 g (66%) of ether 2 as a fraction boiling at 53-71В°C/29 Torr, purity ~97%. Redistillation gave 101 g of ether 2, b.p. 62-64В°C/29 Torr (lit. data: b.p. 54В°C/20 Torr [1]).
References
- I.L.Knunyants, B.L.Dyatkin, Izv. Akad. Nauk, ser.khim. 1958, p.633 (Eng.Trans.)
- C.G.Krespan, D.C.England, J.Am.Chem.Soc., 1981, 103, 5598
- C.G.Krespan, D.A.Dixon, J.Org.Chem., 1986, 51, 4460
- I.Wlassics, V.Tortelli, S.Carella, C.Monzani, G.Marchionni, Molecules, 2011, 16, 6512
- A.P.King, C.G.Krespan, US Pat. 4384128 (1983).
Recommended for publication by Prof. S. Sterlin
Fluorine Notes, 2013, 86, 9-10
