Received: December , 2012
Fluorine Notes, 2013, 86, 1-2
Substitutive Bromination of Perfluorotoluene with Aluminium Bromide
N.D.Kagramanov, I.V.Anayev, V.F.Cherstkov, S.R.Sterlin
aA.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences, ul. Vavilova 28, V-334, GSP-1, 119991 Moscow, Russia
e-mail: sterlins@yandex.ru
Abstract: It is shown that along with the formation of 4-bromoperfluororoluene and 2,4-dibromoperfluorotoluene as the main products of the reaction of perfluorotoluene with aluminium bromide the isomeric tribromodifluorobenzotrifluorides and 4,4'-dibromooctafluoro-О±,ОІ -dibromostilbene are also formed as minor components of the reaction mixture. Aluminium bromofluoride formed in situ is presumably a catalyst of isomerization of intermediate pentafluorobenzotribromide into corresponding brominated derivatives of benzotrifluoride.
Keywords: 4-bromoperfluororoluene; 2,4-dibromoperfluorotoluene; aluminium bromofluoride.
Earlier it has been found that gradual addition of AlBr3 to boiling perfluorotoluene (1) led to the formation of p-bromotoluene (2) as the only reaction product [1]. Detailed study of this reaction demonstrated that under more mild conditions (pot temperature <45В°C) pentafluorobenzotribrimide (3) was the initial product, which transformed into a mixture of 2 and p-bromotetrafluorobenszotribromide (4) in the ratio 2:4 = 5:1 under prolonged refluxing of the reaction mixture (8h) [2].
In order to obtain di- and tribromotoluenes containing bromine atoms in aromatic moiety - as potential starting materials for preparing the corresponding di- and trihydrofluorotoluenes (the reduction of perfluoroarylhalogenides to the corresponding perfluoroarylhydrides was described in [3,4]) - we have attempted to achieve deep bromination of perfluorotoluene under the action of AlBr3 without affecting CF3-group.
It’s obvious that the migration of bromine atom from benzyl to aromatic position proceeds with the participation of benzyl carbocations A, generated under the action of aluminium halogenides (cf. [2]), but unlike perfluorobenzyl carbocation in which positive charge is located in o- and p-positions of the aromatic ring, charge localization in bromo- and dibromoperfluorobenzyl cations is not so evident due to +M-effect of bromine atoms in aromatic moiety.
Indeed the quantum-chemical calculation of a number of dibromopentafluorobenzyl cations and subsequent analysis of integral atomic characteristics obtained within the frame of R. Bader’s theory “Atoms in Molecules” doesn’t give any preference in favor of any definite brominated products from the point of view of atomic charges distribution. Thus the absolute values of aromatic C-atoms charges are almost totally determined by the nature of the nearest substituent: the average charge value for C(F) is +0.68е and for C(Br) -0.03е.
From the other hand the insignificant variation of the charge values of C(F)-atoms should be formally determined by mesomeric effects. Indeed the most electron-deficient centre in 2,4-dibromopentafluorobenzyl cation is located at C(F)-atom in the fifth position of aromatic ring, though its charge is higher than average value for C(F)-atoms on 0.01e only; among all C(F)-atoms in this cation the lowest positive charge is observed at C-atom in the third position (+0.67Рµ). The charge distribution obtained for 2,4-dibromocation is in a good agreement with total energy values (thermodynamic stability) of tribromotoluenes 6-8 (see below).
We have established that the reaction of 1 with AlBr3 under the conditions of preparing p-bromoperfluorotoluene [1], but provided that the relative content of AlBr3 was increased from 13 up to 52.5% resulted in the formation of p-bromotoluene 2 and o,p-dibromotoluene 5 as the main reaction products (the yields 39 and 16.3% respectively based on 1 taken in the reaction) along with a complex mixture of minor components 5a-10. The following compounds were identified according to CMS-data: m,p-dibromotoluene 5a, isomeric tribromobenzotrifluorides 6-8 in a ratio 6:7:8 = 3:1:0.1, o,p-dibromoperfluorobenzylbromide (9) and a mixture of E,Z-isomers of 4,4’-dibromooctafluoro- α,β -dibromostilbene (10).
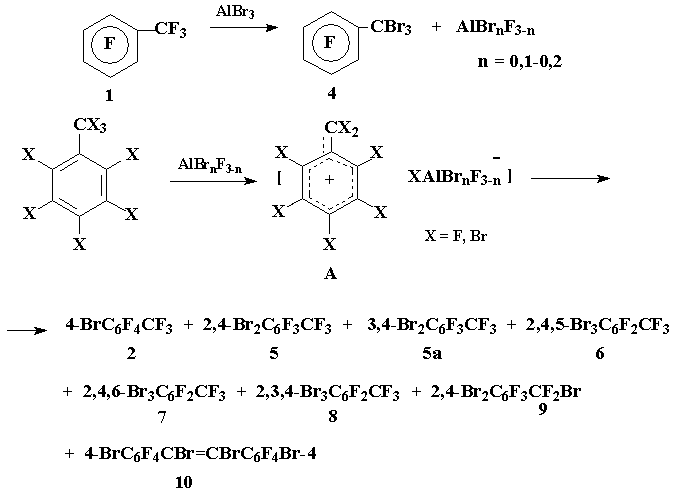
The bromination of perfluorotoluene gives p-bromoperfluorotoluene 2 as the only reaction product whereas the bromination of toluene 2 leads to the formation of dibromotoluene 5 and its isomer 5a as well in a ratio 5:5a = 20:1, that is rather connected with competitive localization of electron-deficient centre in m-position of p-bromoperfluorobenzyl cation as a result of electron-donating influence of bromine atom. The formation of isomeric tribromides 6-8 – the products of further bromination of dibromotoluenes 5-5a – evidently indicates at the absence of clear-cut distribution of positive charge in o,p-dibromoperfluorobenzyl cation that is determined by competitive influence of electronic and steric factors: on the one hand the electron withdrawing influence of CF2-group and +M-effect of two bromine atoms and on the other hand steric hindrances created by such bulky substituents as difluoromethylene and two bromine atoms.
In fact, according to quantum-chemical calculations the relative thermodynamic stability of tribromobenzotrifluorides 6-8 decreases in the row 6>7>8, being irrespective of the calculation method type and a choice of the basis set. Herein the difference in total energies of isomers is sufficiently small and changes slightly upon transition from the pair 6-7 (1.9 kkal/mol) to the pair 7-8 (0.9 kkal/mol), that most likely indicates at the competitive influence of steric and electronic effects of the substituents and at the qualitative level confirms CMS-data (6:7:8 = 3:1:0.1). Here it is worth noting the seeming quantitative disagreement between the ratio of the compounds 6-8 according to CMS-data and the ratio of calculated total energies of these isomers. Obviously, such difference can be explained by a number of complicated factors of real medium, especially the effects of specific and nonspecific solvation leaving from account in our calculations.
It was mentioned above that benzotribromide 3 is an intermediate of the reaction of 1 with AlBr3 that leads to the formation of toluene 2. The compound 3 can be readily obtained in 56% yield from equimolecular mixture of 1 and AlBr3 at 20°С (the yield of compound 3 can be increased up to 80% if the excess of perfluorotoluene is used (see experimental)) [2]. Nevertheless we failed to obtain toluene 2 by the reaction of 1 with 3 in the presence of catalytic amounts AlBr3 (mol. ratio 1:3:AlBr3 = 67:33:1; ~112-115°C/5 h).
It can de assumed that the dependence of the reaction on the quantity of AlBr3 and the reaction conditions is connected with the degree of substitution fluorine atoms for bromine ones in the catalyst. When aluminium bromide is gradually added to the excess of perfluorotoluene at ~35-40°C under stirring only exchange reaction with participation of fluorine atoms in benzyl position takes place that results in the formation of benzotribromide 3 and aluminium fluoride – a polymer, in which coordination number of aluminium is 6 [5], and hence it’s unable to perform a role of Lewis acid, capable to generate carbocations.
At the same time it was shown in a number of works that aluminium fluorohalogenides of general formula AlF3-xHalx [Hal = Cl (ACF), Hal =Br (ABF); x = 0,1-0,3] that obtained by exchange reaction of the corresponding aluminium trigalogenides with fluorochloromethanes, preferably with CFCl3 are more power Lewis acids in comparison with AlCl3 or AlBr3 [6-10]. Thus according to [8] ABF and ACF synthesized by this technique have the structure of polyoctahedrons in which chlorine and bromine atoms carry bridge function that leads to the elongation of Al-Hal-bond and as a consequence to the increasing of positive charge at Al-atom in comparison with AlF3. The higher activity of ABF than AlBr3 as Lewis acid was confirmed experimentally by easy isomerization of 1,2-dibromohexafluoropropane into 2,2-dibromohexafluoropropane [8].
The experimental data obtained allow coming to the conclusion, that isomerization of primarily formed pentafluorobenzotribromide 3 into bromobenzotrifluorides is achieved under the action of ABF but not AlBr3. The absence of stirring and usage of relatively large quantities of AlBr3– ≤ 10-15 mol % to perfluorotoluene – favour the formation of ABF in the form of relatively big aggregated particles, conserving its activity during several hours. If these conditions are not kept sufficiently fast formation of polymeric AlF3 occurs in the presence of such active source of fluoride anion as perfluorotoluene. Taking into consideration that this compound is a weak Lewis acid it can be hardly a catalyst of the reactions under the question.
Experimental
1H, 19F NMR spectra were recorded on Bruker AVANCE-300 spectrometer at 300 and 282 MHz, accordingly; the external standard was CDCl3 or D2O. Chemical shifts for 1H spectra are presented vs. the residual signal of the solvent (Оґ 7.25; 4.79) and are given in ppm vs. tetramethylsilane. Chemical shifts in 19F spectra are given in ppm vs. CF3CO2H. Downfield shifts are positive. The mass spectra were recorded on Finnigan Polaris Q (Trace GC ultra) Mass spectrometer.
Quantum-chemical calculations
Quantum-chemical modeling of tribromosubstituted perfluorotoluenes and corresponding dibromosubstituted benzyl cations was carried out in GAUSSIAN 03 program package [11] using different DFT functionals and standard triple split basis sets (results obtained by m06-2x [12] / 6-311++G** calculation are discussed). During optimization of molecule parameters standard convergence criteria for forces and atomic shifts were used. All models obtained correspond to the ground state: a type of each stationary point was determined by calculations of matrix of potential energy second derivatives. The topological analysis of the charge density distribution function (ρ(r)) and consequent ρ(r) integration over atomic basins were performed using AIMAll program package [13]. During the integration the absolute value of 1/4∇2ρ(r) was not more than 6×10-3 for the whole molecule.
The reaction of perfluorotoluene with AlBr3
Aluminium bromide (15.6 g, 58 mmol) was added portion wise during 1 h to the boiling perfluorotoluene 1 (26 g, 110 mmol) (mol. ratio 1:AlBr3 = 1.89:1), the reaction mixture was kept under reflux 3h (pot temperature 160-165°C, evolution of Br2), cooled to ~25°C, extracted with boiled CH2Cl2, extract was filtered, the solvent and 1 that didn’t enter the reaction were distilled off (pot temperature ~135°C). According to CMS-data the residue contained compounds 2 and 5 as the main reaction products and compounds 5a (5a:5 = 1:20), 6-10 as minor components. Further rectification afforded 22 g of a fraction (42-105°C/10 Torr) that contained 88% of compounds 2 and 5 (2:5 = 2:1) (GLC); the yields of 2 and 5 39,5 и 16,3% respectively. The bromide 2 was identified by comparison (GLC; 19F NMR) with authentic sample obtained by method [1]. The analytical sample of 2,4-dibromoperfluorotoluene 5 (b.p. 77-79°C/8 Torr) was isolated by rectification. Found %: C 23.38; F 31.77; Br 44.76. C7Br2F6. Calc. %: C 23.46; F 31.84; Br 44.69. Spectrum 19F NMR: -19.10 (3Fα), d, JFα-F6 = 33.51 Hz; 18.56 (1F3), d, JF3-F6 = 11.37 Hz; 47.17 (1F5), d, JF5-F6 = 20.94; 59.11 (1F6), ddq. Mass-spectrum 5 (m/z, reference): 356 [M]+ 100; 337 [M-F]+ 15; 306 [M-CF2]+ 20; 277 [M-Br]+ 45; 258 [M-Br-F]+ 6; 227 [M-Br-CF2]+ 3; 208 [M-Br-CF3]+ 10; 198 [M-2Br]+ 80; 179 [C7F5]+ 55; 160 [C7F4]+ 8; 148 [C6F4]+ 40; 141 [C7F3]+ 11; 129 [C6F3]+ 25; 117 [C5F3]+ 10; 110 [C6F2]+ 11; 98 [C5F2]+ 11; 93 [C3F3]+ 6; 79 [C5F]+ 12; 69 [CF3]+ 7.
The structures of compounds 5a-9 were suggested according to CMS-data. Herein it has been taken into consideration that ionization of bromine containing derivatives of benzotrifluoride affords molecular ions, whereas the compounds containing bromine atoms in О±-position form ions [M-Br]+.
Mass-spectrum 3,4-Br2C6F3CF3 (5a) (m/z, reference, %): 356 [M]+ 0.5; 277 [M-Br]+ (100%); 258 [M-Br-F]+ 1; 198 [M-2Br]+ 40; 179 [C7F5]+ 32; 148 [C6F4]+ 8; 141 [C7F3]+ 3; 129 [C6F3]+ 10; 117 [C5F3]+ 5; 98 [C5F2]+ 5; 79 [C5F]+ 4.
Mass-spectrum 2,4,5-Br3C6F2CF3 (6) (m/z, reference, %): 416 [M]+ 1,5; 397 [M-F]+ 0,3; 366 [M-CF2]+ 0,5; 337 [M-Br]+ 46; 318 [M-Br-F]+ 0,3; 258 [M-2Br]+ 34; 239 [M-2Br-F]+ 3; 208 [C6BrF3]+ 4; 189 [C6BrF2]+ 1,3; 179 [C7F5]+ 100; 160 [C7F4]+ 10; 148 [C6F4]+ 2; 141 [C7F3]+ 9; 129 [C6F3]+ 25; 110 [C6F2]+ 10; 79 [C5F]+ 10; 69 [CF3]+ 3.
Mass-spectrum 2,4,6-Br3C6F2CF3 (7) (m/z, reference, %): 416 [M]+ 30; 397 [M-F]+ 2; 366 [M-CF2]+ 2; 337 [M-Br]+ 25; 318 [M-Br-F]+ 1; 258 [M-2Br]+ 36; 239 [M-2Br-F]+ 6; 208 [C6BrF3]+ 2; 189 [C6BrF2]+ 5; 179 [C7F5]+ 100; 160 [C7F4]+ 14; 148 [C6F4]+ 3; 141 [C7F3]+ 13; 129 [C6F3]+ 26; 110 [C6F2]+ 17; 79 [C5F]+ 9; 69 [CF3]+ 4.
Mass-spectrum 2,3,4-Br3C6F2CF3 (8) (m/z, reference, %): 416 [M]+ 25; 397 [M-F]+ 2; 366 [M-CF2]+ 2; 337 [M-Br]+ 27; 260 [M-2Br]+ 37; 239 [M-2Br-F]+ 6; 208 [M-2Br-CF2]+ 4; 179 [C7F5]+ (100%); 160 [C7F4]+ 15; 148 [C6F4]+ 3; 141 [C7F3]+ 7; 129 [C6F3]+ 20; 110 [C6F2]+ 19; 93 [C3F3]+ 7; 79 [C5F]+ 9; 69 [CF3]+ 5.
Mass-spectrum 2,4-BrC6F2CF2Br (9) (m/z, reference, %): 337 [M-Br]+ 33; 258 [M-2Br]+ 23; 239 [M-2Br-F]+ 1,3; 179 [C7F5]+ 100; 160 [C7F4]+ 7; 148 [C6F4]+ 3; 141 [C7F3]+ 7; 129 [C6F3]+ 18; 110 [C6F2]+ 6; 98 [C5F2]+ 5; 93 [C3F3]+ 5; 79 [C5F]+ 5; 69 [CF3]+ 3.
Mass-spectrum 4-BrC6F4CBr=CBrC6F4Br-4 (10) (a mixture of E-Z-isomers) (m/z, reference, %): 636 [M]+ 15; 557 [M-Br]+20; 478 [M-2Br]+ 100; 399 [C14BrF8]+ 10; 349 [C13BrF6]+ 6; 320 [C14F8]+ 70; 301 [C14F7]+ 10; 270 [C13F6]+ 35; 251 [C13F5]+ 22; 239 [C7BrF4]+ 11; 220 [C12F4]+ 8; 201 [C12F3]+ 8; 160 [C7F4]+ 27; 141 [C7F3]+ 5; 110 [C6F2]+ 2; 79 [C5F]+ 1; 69 [CF3]+ 1.
The preparation of pentafluorobenzotribromide 3
Aluminium bromide (25 g, 93.6 mmol) was added in 1-1.2 g portions to perfluorotoluene (105 g, 445 mmol) under vigorous stirring for 1.5 h, sustaining the temperature of the reaction mixture at 38-40В°C, the stirring was continued for 1.5 h more, the liquid part of the reaction mixture was decanted, the precipitate was washed with F 113 (2x35 ml), the solvent was evaporated, the residue was combined with the liquid part of the reaction mixture and distilled to give 31,2 g (79%) of tribromide 3, b.p. 92-93,5В°C/2-3 Torr; lit.data: b.p. 110В°C/5 Torr [2]. Mass-spectrum 3 (m/z, reference, %): 337 [M-Br]+ (100%); 258 [M-2Br]+; 17В°9 [C7F5]+; 160 [C7F4]+; 141 [C7F3]+; 129 [C6F3]+; 93 [C3F3]+.
References
- V.F.Cherstkov, S.R.Sterlin, L.S.German, Izv. Acad. Nauk SSSR, Ser. Khim., 1985, # 11, 2647; В Russian Chem. Bul. 1985,(31),11, 2452
- K.V.Dvornikova, V.E.Platonov, G.G.Yakobson, J.Or.Khim., 1991, 27, #3, 609
- D.V.Trukhin, N.Yu.Adonin, V.F.Starichenko, Pat. RF в„– 2152921 (2000)
- V.I.Krasnov, V.E.Platonov, Russ.J.Org.Chem., 2000, 36, # 10, 1488
- F.A.Cotton, G.Wilkinson, “Advanced Inorganic Chemistry”, part 2, p.288, Moscow, “Mir”, 1969 (Russ.Ed.)
- РЎ.G.Krespan, V.A.Petrov, Chem.Rev., 1996, 96, #8, 3269
- РЎ.G.Krespan, D.A.Dixon, J.Fluor.Chem., 77 (1996), 117
- T.Krahl, E.Kemnits, Ang.Chem. Int.Ed., 2004, 43, 6653
- T.Krahl, R.Stoesser, E.Kemnitz, G.Scholz et al, Inorg.Chem., 2003, 42, 6474
- A.Dimitrov, D.Heidemann, E.Kemnitz , Inorg.Chem., 2006, 45, 10807
- Gaussian 03, RevisionВ C.02, M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, J. A. Montgomery, Jr., T. Vreven, K. N. Kudin, J. C. Burant, J. M. Millam, S. S. Iyengar, J. Tomasi, V. Barone, B. Mennucci, M. Cossi, G. Scalmani, N. Rega, G. A. Petersson, H. Nakatsuji, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, M. Klene, X. Li, J. E. Knox, H. P. Hratchian, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, P. Y. Ayala, K. Morokuma, G. A. Voth, P. Salvador, J. J. Dannenberg, V. G. Zakrzewski, S. Dapprich, A. D. Daniels, M. C. Strain, O. Farkas, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. V. Ortiz, Q. Cui, A. G. Baboul, S. Clifford, J. Cioslowski, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. L.
- Y. Zhao, D.G. Truhlar, Theor. Chem. Acc., 2008, 120, 215
- T. A. Keith, AIMAll (Version 08.01.25), 2008, http://aim.tkgristmill.com.
Recommended for publication by Prof. S. Sterlin
Fluorine Notes, 2013, 86, 1-2
