Received: September, 2012
Fluorine Notes, 2012, 84, 5-6
Method for the synthesis of pure (E)-3-(perfluoroalkyl)-allylic alcohols
Peter Ivanko, Csongor Szíjjártó, Dénes Szabó and József Rábai*
Institute of Chemistry, Eotvos Lorand University, P. O. Box 32, H-1518, Budapest 112, Hungary
e-mail: rabai@elte.hu
Abstract:Simple method for the preparation of (E)-3-(perfluoroalkyl)-allylic alcohols (1) is suggested.
Keywords: (E)-3-(perfluoroalkyl)-allylic alcohols, 3-perfluoroalkyl-2-iodo-1-propanols, lethargic dehydrohalogenation.
Perfluoroalkylpropenols have been prepared by the dehydrohalogenation of appropriate perfluoroalkyl-halohydrins and used as precursors for the synthesis of low surface energy materials. Their syntheses usually afford technical grade mixtures of the (E)-:(Z)-isomers, along with 2-perfluoroalkylmethyl-oxirane type side products. These methods call for simple inorganic bases such as NaOH, KOH, NaHCO3, Na2CO3, alkoxides, or the bicyclic amidine type bases like DBU or DBN and use alcohol type solvents (MeOH, EtOH, and i-PrOH) at 15 to 100В°C reaction temperatures for 1-14 h treatments [1-3].
Perfluoroalkylpropenols [(E)-I/(Z)-I] could be made as disclosed in a US Patent in a 93:6 ratio of (E)-:(Z)-I with 69-91% yields using N-methylpirrilidone as solvent and Me4NHCO3 base for dehydrohalogenation of the starting halohydrins [4].
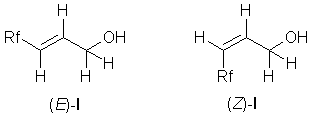
We have found that further increase of the (E)-:(Z)-isomeric ratio of F-alkyl-allylic
alcohols (1a-e) was possible with the selective but rather
slow dehydroiodination reaction of the easily accessible 3-perfluoroalkyl-2-iodo-1-propanols
(2a-e, "fluorous-iodohydrins" [5]) with diethylamine as a base in ether
at room temperature. The title compounds were obtained in high yields and purity after 11 to
44 days of reaction time. Any attempts to increase the rate of this type of reaction by using
forcing conditions resulted in significant drop of yields and isomeric purities (cf. with the
definition of "Lethargic reaction", [6]).
It should be noted that these iodohydrins
(2a-e) could be involved in three different types of HI elimination reaction,
either leading to the title Rfn-allylic alcohols (1a-e), fluorous-oxiranes (RfnCH2CHCH2O)
or -aldehydes (RfnCH2CH2CH=O) [7].
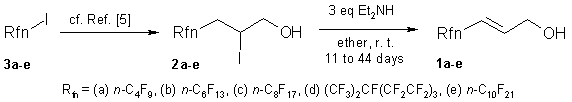
The products obtained here were characterized by 1H-, 13C and 19F-NMR spectra and GC. Their GC analyses indicate 95-97% presence of the trans-isomer, while that of the minor impurities at shorter retention times never exceeds 4%.
Experimental
The starting perfluoroalkyl-iodohydrins 2a-e were prepared by the reaction of perfluoroalkyl iodides 3a-e and allylic alcohol as reported [5]. 1H-, 13C- and 19F-NMR spectra were recorded on Bruker Avance 250 instrument using a 5 mm inverse 1H/13C/31P/19F probe head at room temperature. Chemical shifts (δ) are given in parts per million (ppm) units relatively to the internal standard TMS (δ=0.00 for 1H,δ=0.00 for 13C) and to CFCl3 as external standard (δ=0.00 for 19F). В Melting points were determined on a Boetius micro-melting point apparatus and are uncorrected. The reactions were monitored by gas chromatography (Hewlett-Packard 5890 Series II, PONA [crosslinked methylsilicone gum] 50 m x 0.2mm x 0.5 mm column, H2 carrier gas, FID detection; Program: 120 В°C, 5 min, 10 В°C/min, 250 В°C, 5 min; Inj.: 250В°C, Det: 280В°C).
General Procedure (GP). A solution of the iodohydrin (250mmol) in diethyl ether (2mL for 1g iodohydrin) was mixed with diethyl amine (750mmol) at room temperature. The reaction vessel was flushed with argon, closed and wrapped with an aluminum foil to protect from sunlight. The mixture was kept in the dark at room temperature until 99+% conversions were reached.The solid Et2NH*HI salt was filtered off, washed with ether, and dried. Then the В filtrate was washed with 5% HCl-H2O and water and dried (Na2SO4). The products were isolated by fractional distillation using a short Vigreaux column.
(E)-4,4,5,5,6,6,7,7,7-nonafluoro-hept-2-ene-1-ol (1a). The C4F9-iodohydrin (2a, 100 g, 248 mmol, GC assay 96%) was reacted according to the GP for 11 days and worked up to yield 57.05 g (83%) colorless oil, bp 85-86 В°C/20 mm Hg, with GC purity of 95.8%. 1H NMR (CDCl3) Оґ: 6.52 (hd, 1H, J= 15.8, 2.2 Hz, RfnCH); 5.95 (qa, 1H, J= 15.6 Hz, CHCH2); 4.32 (m, 2H, J= 3.8, 2.2, 1.6 Hz, CH2OH); 2.93 (s, br, 1H, OH); 13C NMR (CDCl3) Оґ: 61.3 (C1); 116.4 (t, C2, J= 23.4 Hz); 141.6 (t, C3, J= 8.7 Hz); 19F NMR (CDCl3) Оґ: -81.95 (tt, 3F, J= 9.9, 3.0 Hz, CF3); -112.58 (~t, 2F, J= 12.0 Hz, CH2CF2); -125.05 (m, 2F, J= 10.1, 6.9 Hz); -126.59 (m, 2F, J= 6.9, 5.2 Hz).
(E)-4,4,5,5,6,6,7,7,8,8,9,9,9-tridecafluoro-non-2-ene-1-ol (1b). The
C6F13-iodohydrin (2b, 200 g, 397mmol, GC assay 96%) was
reacted according to the GP for 14 days and worked up to yield 123 g (82%) colorless oil, bp
103 В°C/20 mm Hg, with GC purity of 96.1%. 1H NMR (CDCl3) Оґ: 6.52 (hd,
1H, J= 15.8, 2.2 Hz, RfnCH); 5.95 (qa, 1H, J= 15.0 Hz, CHCH2);
4.33 (m, br, 2H, CH2OH); 2.66 (s, br, 1H, OH); 13C
NMR (CDCl3) Оґ: 61.5 (C1); 116.6 (t, C2, J= 23.4 Hz); 141.4 (t, C3, J= 8.5 Hz); 19F
NMR (CDCl3) Оґ: -81.77 (tt, 3F, J= 9.9, 2.6 Hz, CF3);
-112.43 (tt, 2F, J= 13.4, 3.4 Hz, CH2CF2);
-122.46
(br, 2F); -123.73 (br, 2F); -124.17 (m, br, 2F); -127.04 (m, 2F)
(E)-4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,11-heptadecafluoro-undec-2-ene-1-ol (1c). The C8F17-iodohydrin (2c, 200 g, 331mmol, GC assay 97%) was reacted according to the GP for 19 days and worked up to yield 140 g (89%) colorless oil, bp 126-127В°C/16 mmHg, with GC purity of 95.5%. 1H NMR (CDCl3) Оґ: 6.52 (hd, 1H, J= 15.8, 2.2 Hz, RfnCH); 5.96 (qa, 1H, J= 15.2 Hz, CHCH2); 4.33 (m, br, 2H, CH2OH); 2.48 (s, br, 1H, OH); 13C NMR (CDCl3) Оґ: 61.5 (C1); 116.6 (t, C2, J= 23.4 Hz); 141.4 (t, C3, J= 8.5 Hz); 19F NMR (CDCl3) Оґ: -81.75 (~t, 3F, J= 9.9 Hz, CF3); -112.40 (~t, 2F, J= 12.9 Hz, CH2CF2); -122.25 (m, br, 2F); -122.75 (m, br, 4F); -123.55 (m, br, 2F); -124.12 (m, br, 2F); -127.00 (m, br, 2F)
(E)-4,4,5,5,6,6,7,7,8,8,9,9,10,11,11,11-hexadecafluoro-10-trifluormethyl-undec-2-ene-1-ol (1d). The iso-C9F19-iodohydrin (2d, 48.3 g, 73.9mmol) was reacted according to the GP for 9 days and worked up to yield 27.7 g (71%) colorless oil, bp 142-144В°C/17 mmHg, with GC purity of 94.4%. 1H NMR (CDCl3) Оґ: 6.52 (hd, 1H, J= 15.8, 2.2 Hz, RfnCH); 5.96 (qa, 1H, J= 14.7 Hz, CHCH2); 4.34 (m, br, 2H, CH2OH); 2.24 (s, br, 1H, OH); 13C NMR (CDCl3) Оґ: 61.6 (C1); 116.6 (t, C2, J= 23.4 Hz); 141.5 (t, C3, J= 8.7 Hz) 19F NMR (CDCl3) Оґ: -72.62 (m, 6F, CF3); -112.31 (m, 2F, CH2CF2); -115.70 (s, br, 2F); -121.46 (s, br, 2F); -122.14 (m, br, 4F); -124.02 (m, br, 2F); -186.80 (m, br, 1F).
(E)-4,4,5,5,6,6,7,7,8,8,9,9,10,10,11,11,12,12,13,13,13-heneicosafluoro-tridec-2-ene-1-ol (1e). The C10F21-iodohydrin (2e, 25.0 g, 41.4mmol) was reacted according to the GP for 44 days and worked up to yield 16.9 g (71%) colorless melt, bp 108-110В°C/0.1 mm Hg, mp 54-56 В°C/isooctane, with GC purity of 94.4%. 1H NMR (CDCl3) Оґ: 6.71 (hd, 1H, J= 15.6, 2.4 Hz, RfnCH); 6.06 (qa, 1H, J= 15.2 Hz, CHCH2); 4.33 (s, br, 2H, CH2OH); 2.94 (s, br, 1H, OH); 13C NMR (CDCl3) Оґ: 61.5 (C1); 115.4 (t, C2, J= 23.4 Hz); 141.5 (t, C3, J= 8.5 Hz); 19F NMR (CDCl3) Оґ: -81.74 (t, 3F, J= 10.4 Hz, CF3); -112.41 (t, 2F, J= 12.9 Hz, CH2CF2); -122.21 (s, br, 2F); -122.60 (s, br, 8F); -123.51 (s, br, 2F); -124.13 (s, br, 2F); -126.99 (m, br, 2F)
Acknowledgement. This study was supported by the Hungarian Scientific Research Foundation (OTKA K 062191 "Sustainable fluorous chemistry"): The European Union and the European Social Fund have provided financial support to the project under the grant agreement no.TÁMOP_4.2.1./B-09/1/KMR-2010-0003
References
- GB1101049, Daikin Kogyo Kabushiki Kaisha, Patent filed: May 19, 1966.
- JP 52144610, A. Yoshio, D. Shigeo, K. Yasuhisa, O. Kazuo, (Daikin Kogyo), 1977.
- US4278552, I. Hisamoto, et al. (Daikin Kogyo, Co.,Ltd) Patent filed: April 12, 1979. (et al. = N. Enjo, C. Maeda, T. Esaka, Y. Omure, T. Iida, H. Aomi)
- US Patent 4754082, K. Raab, J. J. Pöschl, (Hoechst), Patent filed: July 2, 1987.
- J. Rábai J, Cs. Szíjjártó, P. Ivanko, D. Szabó. Synthesis 2007, 2581-2584.
- D. E. Pearson, O. D. Keaton, J. Org. Chem. 1963, 28, 1557-1958.
- P. Ivanko, F. T. Takács, J. Rábai, Convergent synthesis of fluorous reagents. 14thEuropean Symposium on Fluorine Chemistry, Poznań, Poland, 2004. Book of Abstracts, p. 84.
Recommended for publication by S. Igoumnov
Fluorine Notes, 2012, 84, 5-6
