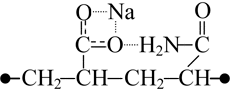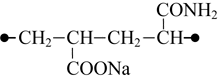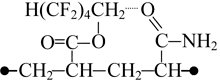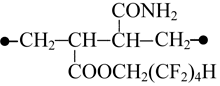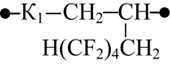Received: July, 2011
Fluorine Notes, 2012, 84, 3-4
The impact of octafluoropentyl fragment on intramolecular and intermolecular interactions within the acrylamide - sodium acrylate copolymer in aqueous solutions
1O.S.Rakhimova, 2R.G.Fedunov
1Volgograd State Technical University Russian Federation, 400005, Volgograd, Lenin ave., 28
e-mail: organic@vstu.ru
2Volgograd State University, prospect Universitetsky, 100, Volgograd, 400062
e-mail:
rofedor@yandex.ru
Abstract: Ab initio quantum-chemistry method with 6-31G basis set is applied in the analysis of both intramolecular and intermolecular interactions of the elemental chain links both for copolymers modified with an octafluoropentyl fragment and for their complexes with water molecules. It is found that among all possible conformations of the copolymer links the most energetically beneficial is the one that favors the formation of globular structures. The introduction of octafluoropentyl groups results in equally probable formation of linear structures. An explanation is offered for the downtrend in the viscosity of polyfluoroalkylated copolymer aqueous solutions.
Keywords: Copolymer, acrylamide, sodium acrylate, viscosity of aqueous solutions, polyfluoroalkylated copolymer, quantum-chemical calculations.
It is well-known [1-5], that both intra- and intermolecular interactions with the participation of macromolecular systems in solutions are driven by the conformational alterations of the dissolved polymer macromolecules. It was also marked that the polar functional groups if available in the macromolecular chain units produce mutual electrostatic effect and influence also on the solvent molecules. Moreover, sometimes there are effects such as increase in viscosity upon dilution of the copolymer, for example, in solutions of polymethacrylic acid and methyl methacrylate in dimethylformamide or acetone, which is explained by a significant increase in the ionization of macromolecules [4, 5].
It was shown [6] that the introduction of octafluoropentyl fragment by substitution of a sodium atom in the carboxylate group of acrylamide/sodium acrylate copolymer impacts on such an important property of copolymer water solutions as their viscosity, which is determined by the solution discharge time. At room temperature the discharge time of the copolymer 0.3% aqueous solution drops from 83 s (copolymer without polyfluorinated modifier to 16 s (copolymer with modifier), while that of 0.1% solution drops from 59 s to 10 s.
In this our study the quantum-chemical ab-initio method with 6-31G basis sets [7] is applied for the analysis of both electronic structure and geometry of the fragments of acrylamide/sodium acrylate copolymer for various combinations of its chain units, and for similar combinations after the introduction of an octafluoropentyl fragment, and for their associates with water molecules as well. The mutual influence is studied of intramolecular interactions between polar amide or carboxylated groups in chain units and the conformation structure of fragments, and associative interaction between those groups and water molecules.
At the first step we considered various combinations of amide and sodium carboxylate groups within a chain fragment of the copolymer that consists of two monomer-forming elements: amide and sodium carboxylate groups, when being located on the same side of the chain or opposite sides; the introduction of the octafluoropentyl fragment was conducted by the substitution of a carboxylated group for an ester group with an octafluoropentyl fragment in it (table 1).
Table 1. The results of quantum-chemical calculations of copolymer units in various combinations.
| # | Structure combination of the chain units | Designation | Total energy, E0 kcal/mole | Length of carbon chain, nm | Dipole moment, D |
|
1 |
|
K1 |
-612635.7 |
0.4403 |
8.01 |
|
2 |
|
K2 |
-612614.7 |
0.5723 |
11.86 |
|
3 |
|
K3 |
-612621.5 |
0.6086 |
10.26 |
|
4 |
|
K4 |
-1129902.8 |
0.4874 |
6.97 |
|
5 |
|
K5 |
-1129903.7 |
0.6063 |
6.65 |
• end groups are the remains of CH3OOCH2OH radical initiator. The chain length is the distance between oxygen atoms of CH3O- and -OCH2OH end groups.
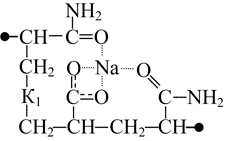
In case that both amide and sodium carboxylate groups are located at the same side of the chain unit in original copolymer (without polyfluoroalkyl substituent) the interaction of those functional groups may reach its maximum. The most energy-efficient structure is K1 combination (table 1); where two oxygen atoms belonging to the sodium carboxylate group and carbonyl oxygen belonging to the amide group interact with the sodium atom. The distance between sodium and each of those three oxygen atoms is nearly the same and varies between 0.224 and 0.233 nm. Each of the oxygen atomic charge is about -0.76, while sodium atomic charge is +0.78. In the said structure the distance between the chain ends is minimal, and the dipole moment is minimal as well. Both spatial positions of the carbon chain atoms and the interaction between those polar groups point to the proneness of the said combination to form globular structures.
The interaction between hydrogen atom of the amide group and oxygen atom of the sodium carboxylate group within K2 combination is less preferable as to compare with K1 combination. Therewith the distance between hydrogen atom of the amide group and oxygen of the sodium carboxylate group achieves 0.194 nm (oxygen atomic charge is -0.831, and hydrogen atomic charge is +0.465), thus violating the symmetry of oxygen atom charge in carboxylated group and shifting sodium to the opposite oxygen of the carboxylated group.
In the case that amide and sodium carboxylate groups are located at different sides of the unit (combination K3) the said interaction is not observed. The sodium atom is then charged +0.82 and located symmetrically to each oxygen atom of the carboxylated group at the distance of 0.22 nm. The charges of carboxylate oxygen atoms are very close, being -0.793 and -0.780. The combination total energy is 6 kcal/mole less, than that of K2 combination.
The spatial location of carbon chain atoms in K2 and K3 combinations is linear. The distance between chain ends for those combinations exceed that in K1 combination by ~0.15 nm. Yet K1 combination is more efficient being susceptible to globular structure formation. Thus upon the introduction of the octafluoropentyl fragments, the comparison was made between two different combinations with the best total energies that varied, however, in the location of carboxylated and amide groups positions relative to the chain units.
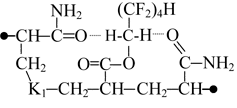
When sodium is introduced instead of the octafluoropentyl fragment the structure polarity decreases, the fact manifests itself by considerable drop of the dipole moment (table 1, K4 and K5 combinations). In the case of K4 combination the distance between its chain ends is 0.047 nm longer than that in K1 combination, while it stays virtually unchanged in more linear combinations (table 1, K3 and K5 combinations). K4 and K5 combination structures differ in the location of their functional groups, but similar in their total energies and nearly similar in their polarities. This leads to the conclusion about equiprobable formation of K4 or K5 structures upon the introduction of octafluoropentyl fragment.
At the next step the calculations were performed with a large number of the copolymer chain units (table 2). The initial structure based on the results of the calculations for K1 and K4 combinations, supplemented, however, in these calculations with one unit containing sodium carboxylate group and two more units containing amide groups. The calculations have shown that the tendency to form globular structures remains for enlarged number of chain units. For example, in the case of 5 chain units the sodium atom may interact with two additional amide groups (table 2, example 3).
If the sodium atom is replaced with an octafluoropentyl fragment the carbon chain length tends to decrease slightly due to associative interaction of mobile hydrogen atoms belonging to CH2 of a polyfluorinated group with oxygen atoms in amide groups. As the chain length increases the dipole moment value drops as to compare with that of initial K1 and K4 structures.
Table 2. The results of quantum-chemical calculations for the fragments of copolymer with 3 or 5 units.
|
# |
Structure combination of chain elements |
Number of units |
Total energy, E0 kcal/mole |
Length of carbon chain, nm |
Dipole moment, D |
|
1 |
|
3 |
-880516,4 |
0.6298 |
3.71 |
|
2 |
|
3 |
-1397779,2 |
0.6004 |
3.72 |
|
3 |
|
5 |
-1188945,5 |
1.0412 |
4.47 |
|
4 |
|
5 |
-1706202,1 |
0.9811 |
6.18 |
We further investigated the associative interactions between the fragments of the original copolymer and water molecules exemplified by K3 combination. We started with the calculations of a complex formed by a unit of the copolymer with one molecule of water oriented towards the sodium carboxylate group. Then we performed calculations for a complex with two molecules of water. The second water molecule was oriented towards the amide group.
Our calculation results evidenced the formation of stable six-membered cyclic associates with structures as shown below (Fig. 1), where the molecules of water undergo polarization. When so doing the water molecule polarization by sodium carboxylate exceeds considerably that by amide group. In water molecule the oxygen atomic charge drops from its initial value -0.81 (monomolecular calculation) to -0.93 due to the interaction with sodium carboxylate group and to -0.89 due to the interaction with amide group.
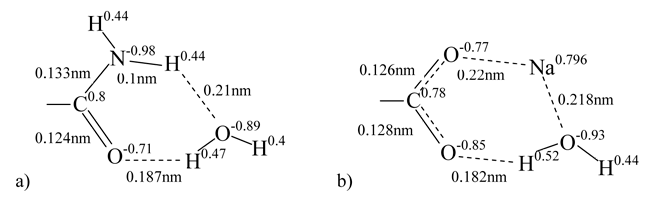
Figure 1. Complexes containing sodium carboxylate (a) or amide (b) groups and water molecules.
Adding of a water molecule to sodium carboxylate group results in strong association between the water molecule hydrogen atom and oxygen atom of the carboxylated group (distance is 0.182 nm). When so doing the symmetric structure of the sodium carboxylate group is disturbed, and the water molecule oxygen begins interacting with sodium that is why the sodium atomic charge decreases (Fig. 1a). The addition of water molecule to amide group weakens the association between the water molecule hydrogen and the carbonyl group oxygen. The atomic charges on hydrogen atoms of NH2 group remain virtually unchanged. Therefore, the impact of water molecule on sodium carboxylate group is stronger than that on amide group.
The interaction between fragments of the modified copolymer and water molecules was studied on the example of K5 combination. Our calculations have shown that associative interactions are possible between carbonyl oxygen of the ester group and hydrogen atom of water molecule, and also interactions are possible between the water oxygen atom and α-hydrogen atom of the methylene group influenced simultaneously by two electron-acceptor groups: perfluorinated carbon chain and carboxylated group. However, this interaction is much weaker than that between sodium carboxylate and amide group. In this case the polarization of water much less and the interacting atoms of water molecule are further from the polar centers (Fig. 2).
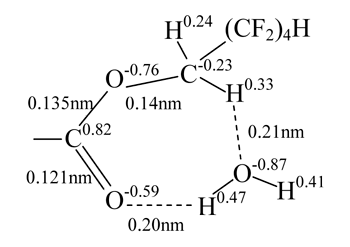
Fig. 2. Complex containing an ester group with octafluoropentyl fragment and water molecule.
The copolymer units participate actively in the formation of complexes with water molecules. From Table 3 one may see that the energy of complex formation with the participation of the copolymer sodium carboxylate groups exceed that of associative interactions between water molecules, the latter, by the results of our calculations performed for water dimer is 8 kcal/mole. The energy of complex formation with the participation of amide group and water molecule achieves 11-12 kcal/mole that is comparable with above mentioned value.
Table 3. The results of quantum-chemical calculations performed for complexes of water with the copolymer units in various combinations.
|
N of combination |
Total energy of the complex, E0c, kcal/mole |
Energy of complex formation, ΔEc, kcal/mole |
Dipole moment, D |
|||
|
1 molec. of water |
2 molec. of water |
1 molec. of water |
2 molec. of water |
1 molec. of water |
2 molec. of water |
|
|
K1 |
-660341.3 |
-708033.8 |
-24.1 |
-35.1 |
7.55 |
6.56 |
|
K3 |
-660325.9 |
-708019.5 |
-22.9 |
-35.0 |
9.74 |
9.24 |
|
K4 |
-1177593.0 |
-1225286.2 |
-8.7 |
-20.4 |
8.03 |
6.58 |
|
K5 |
-1177591.5 |
-1225286.7 |
-6.3 |
-20.0 |
4.72 |
3.60 |
•total energy of water molecule E0w = -47681.5 kcal/mole, energy of interaction for the complex with one molecule of water ΔEc = E0c – (E0+E0w), energy of interaction for the complex with two molecules of water ΔEc = E0c – (E0+2E0w)
The introduction of octafluoropentyl substituent decreases considerably, to 6.3 - 8.7 kcal/mole, the energy of the water molecule interaction with the copolymer functional groups, this evidences of the fragment hydrophoby. Besides of that, the octafluoropentyl substituent located at the opposite side of amide group has considerable impact on the reduction of the dipole moment of the formed complexes (table 3). In the case of complex with two water molecules the dipole moment value reduces by three times. From the comparison of total energy for complexes in K4 and K5 combinations one may see that the introduction of octafluoropentyl fragment makes the linear structure of carbon chain of the copolymer unit in the complex formed with 2 water molecules more preferential.
Therefore, the results of quantum-chemical calculations for K1, K2 and K3 combinations show that the unit chains of non-modified copolymers may form globular structures with very high probability. In the presence of octafluoropentyl fragment the formation of stable polar complexes with water molecules, thus resulting in considerable energy benefit.
The introduction of octafluoropentyl fragment into the copolymer unit results in the decrease in its polarity, and different conformations K4 (globularя) and K5 (linear) become equiprobable. When so doing the addition of water molecule in K4 and K5 combinations makes linear structure more energy-efficient than the globular one, resulting also in considerable decrease of the complex polarity and in less gaining in the energy of complex formation.
As in water solutions the globular-structured macromolecules have higher viscosity than those with robust or flexible linear structures [8], such factors as hydrophoby of octafluoropentyl group together with conformation properties of the complexes of the modified copolymer units in water that govern the polymer chain flexibility, explain the loss in the viscosity of aqueous solutions of polyfluoroalkylated copolymer.
References
- Fuoss R. M. Polyelectrolytes. II. Poly-4-vinylpyridonium chloride and poly-4-vinyl-N-n-butylpyridonium bromide // J. Polymer Sci. / R. M. Fuoss, U. P. Strauss. – 1948. – V3. – P.246-263
- Fuoss R. M. Electrostatic interaction of polyelectrolytes and simple electrolytes. // J. Polymer Sci. / R. M. Fuoss, U. P. Strauss. – 1948. – V3. – P.602-603
- Fuoss R. M. Polyelectrolytes. III. Viscosities of n-butyl bromide addition compounds of 4-vinylpyridine-styrene copolymers in nitromethane-dioxane mixtures† // J. Polymer Sci. / R. M. Fuoss, G. I. Cathers. – 1949. – V4. – P.97-120.
- Eizenberg G. Fuoss R. Nekotorye problemy sovremennoj ehlektrokhimii // M., Izdatinlit – 1958.
- Barabanov V. P. Poliehlektrolity. Izbrannye trudy // Moskva.– 2001.
- Rezul'taty issledovanij sotrudnikami kafedry organicheskoj khimii VolgGTU. / Rahimov A.I., Miroshnichenko A.V., Vershinin D.A., – 2011.
- M.W.Schmidt // J.Comput.Chem. / M.W.Schmidt, K.K.Baldridge, J.A.Boatz, S.T.Elbert, M.S.Gordon, J.H.Jensen, S.Koseki, N.Matsunaga, K.A.Nguyen, S.J.Su, T.L.Windus, together with M.Dupuis, J.A.Montgomery. – 1993. V.14, P.1347-1363.
- Volkov V.A. Kolloidnaya khimiya. Poverhnostnye yavleniya i dispersnye sistemy. // Moskva. – 2001.
Recommended for publication by Prof. Alexander I. Rakhimov
Fluorine Notes, 2012, 84, 3-4