Received: July, 2012
Fluorine Notes, 2012, 83, 7-8
QUANTUM-CHEMICAL INVESTIGATION OF THE REACTION OF DIETHYL CHLOROMALONATE WITH POTASSIUM FLUORIDE
O.S. Andrienko 1,2 , V.V. Zhuk 1, N.F. Mingalimov 1, O.B. Sambueva 1,2, V.I. Sachkov 1,3, A. N. Tretyakov 1, V.A. Yanovsky 1
1 Siberian Physico-technical Institute named of the academician V.D. Kuznetsov of Tomsk State
University, 634050, Russia, Tomsk, Novosobornaya sq.1, tel. (3822)412319,
e-mail: itc@spti.tsu.ru
2 Institute of Atmospheric Optics named of V.E. Zuev Russian Academy of Sciences, Siberian Branch, 6340211, Russia, Tomsk, Academician Zuev sq. 1
3 Institute for Problems of Chemical and Energetic Technologies Russian Academy of Sciences, Siberian Branch, 659322, Russia, Biysk, st. Socialisticheskaya, 1
Abstract: With the quantum-chemical methods the reaction of diethyl 2-chloromalonate with potassium fluoride has been investigated in the gas phase and in solvent using the model of PCM. The way of the reaction has been studied by mechanisms of mono-and bimolecular substitution, the dependence of energy barriers on the polarity of the reaction medium has been established.
Keywords: diethyl 2-fluoromalonate, diethyl 2-chloromalonate, potassium fluoride, quantum-chemical calculations.
The practical importance of diethyl fluoromalonate (DEFM) is caused by its use as fluorine-containing building-block for the production of biologically active monofluorinated heterocyclic compounds, for example, 2-fluorocarboxylicacids, 5-fluorobarbituricacidanditsderivatives [1-3]. At present the well- known methods for obtaining 2-diethyl ether of fluoromalonic acid are not without disadvantages such as low values of a yield of the desired product, harsh conditions of synthesis (the use of toxic and corrosive chemicals) [4-9]. As the interest to fluorinated organic compounds is continuously increasing, it is necessary to develop new methods of synthesis, which could remove the mentioned disadvantages. One of the traditional methods of obtaining organofluorine compounds is the nucleophilic substitution of halogen in the corresponding chlorine and bromine derivatives. Metal fluorides are mainly used as a reagent, which allows to exchange chlorine atom by fluorine, generally potassium fluoride [10].
Previously we have developed the method for the synthesis diethyl fluoromalonate (DEFM) by the action of potassium fluoride on diethyl chloromalonate (DEChM) in aprotonic solvents [11].
For more detailed study of this process the theoretical calculations of the substitution reaction of chlorine with fluorine in diethyl chloromalonate were carried out. The calculations were carried out by methods of density functional (DFT) b3lyp in the basis 6-311 * as the approximation of isolated molecules (gas phase), and in solutions using the method of PCM. The value of a dielectric constant (ε) of the solvent is as a criterion which characterizes its. The error of this method is ± 3 kJ / mol.
We have calculated the thermodynamics of the process of substitution of chlorine by fluorine in diethyl chloromalonate in the gas phase and in solution (Fig. 1, Table. 1) as follows:
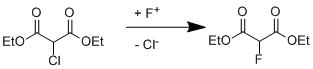
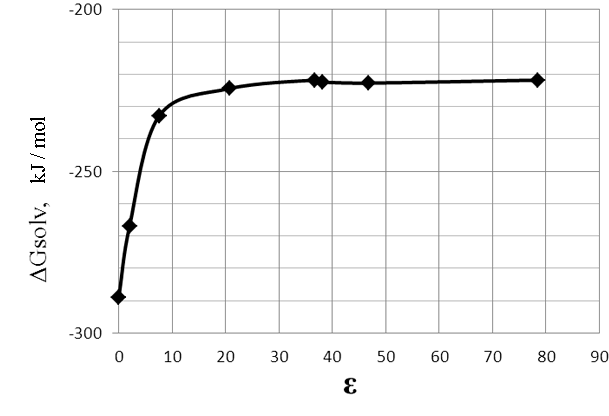
Fig.1.The dependence of ΔG of the reaction (1) on the dielectric constant (ε) of the solvent.
Table 1. The change ΔG the reaction (1) depending on the dielectric constant (ε) of solvent.
|
Solvent |
ε |
ΔGsolv, kJ / mol |
|
Gas phase |
0 |
-289 |
|
Cyclohexane |
2,02 |
-266,9 |
|
Tetrahydrofuran |
7,58 |
-232,8 |
|
Acetone |
20,70 |
-224,4 |
|
Acetonitrile |
36,64 |
-221,8 |
|
Nitromethane |
38,20 |
-222,4 |
|
Dimethylsulfoxide |
46,70 |
-222,7 |
|
Н2О |
78,39 |
-221,8 |
As seen in Figure 1 and Table 1, the reaction is strongly exothermal, with ΔG of the reaction increases with the increase of polarity of the medium.
Also, we have calculated the energy barriers for this reaction by SN1 and SN2 mechanisms in the gas phase and in solvents.
The limiting stage of the reaction by mechanism SN1 is the dissociation of diethyl chloromalonate to cation (Scheme 1, stage I):
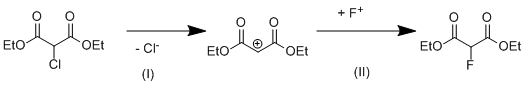
Scheme 1. Monomolecular mechanism of nucleophilic substitution (SN1).
It was found that energy barrier of the reaction is positive and high enough (Fig. 2).
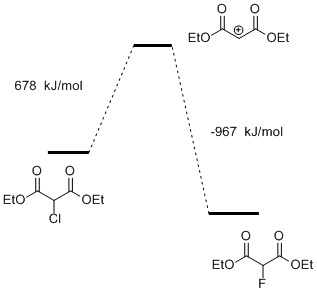
Fig.2. The energy profile of the substitution reaction of chlorine by fluorine in diethyl chloromalonate by the mechanism of SN1 of the reaction (2) in the gas phase.
With the increase of polarity of the medium ΔG of the reaction (2) decreases slightly (Table 2, Figure
3). For carrying out the reaction by mechanism of SN1, even in polar solvents need a high heat to
overcome the high energy barrier.
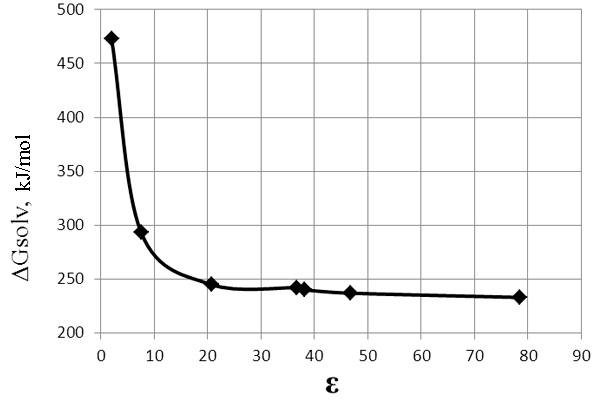
Fig.3. The dependence of ΔG of thereaction (reaction 2, stage I) on the dielectric constant (ε) of solvent.
Table 2. Thedependence of ΔG of the reaction (2) (stage I) on the dielectric constant (ε) of solvent.
|
Solvent |
ε |
ΔGsolv, kJ / mol |
|
Cyclohexane |
2,02 |
473 |
|
Tetrahydrofuran |
7,58 |
293 |
|
Acetone |
20,70 |
245 |
|
Acetonitrile |
36,64 |
242 |
|
Nitromethane |
38,20 |
240 |
|
Dimethylsulfoxide |
46,70 |
237 |
|
Н2О |
78,39 |
233 |
The second stage of the reaction (2) is thermodynamically very biased towards the formation of products
and does not influence the reaction course. By increasing polarity of the medium ΔG of the reaction
(2) is reduced, but not significantly (Fig. 4, Table. 3).
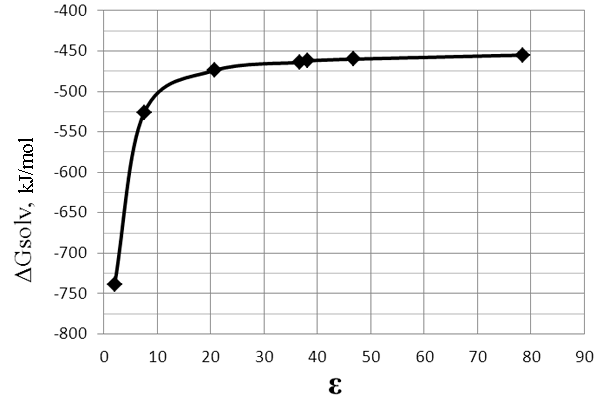
Fig.4. The dependence of ΔG of the reaction (2)(stage II) on the dielectric constant (ε) of solvent.
Table 3. The dependence of ΔG of the reaction (2) (stage II) on the dielectric constant (ε) of solvent.
|
Solvent |
ε |
ΔGsolv, kJ / mol |
|
Cyclohexane |
2,02 |
-739 |
|
Tetrahydrofuran |
7,58 |
-526 |
|
Acetone |
20,70 |
-474 |
|
Acetonitrile |
36,64 |
-464 |
|
Nitromethane |
38,20 |
-462 |
|
Dimethylsulfoxide |
46,70 |
-460 |
|
Н2О |
78,39 |
-455 |
For calculation of the energy barrier of the reaction by the mechanism of the bimolecular substitution on the reaction (3) the transition state has been calculated (Fig. 5).
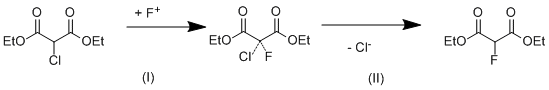
Scheme 2. Bimolecular mechanism of nucleophilic substitution (SN2).
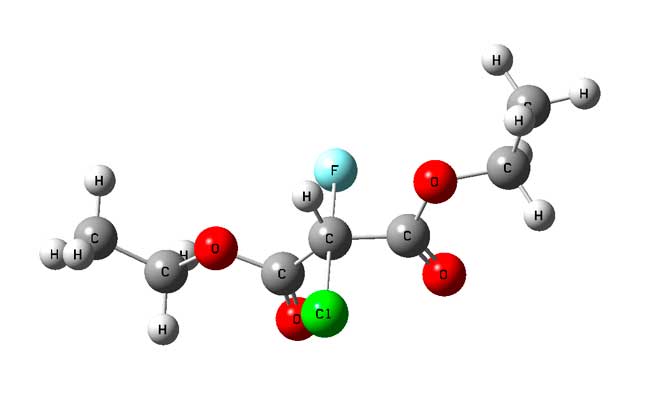
Fig.5. The structure of the transition state of the substitution reaction of chlorine by fluorine in diethyl chloromalonate by the mechanism of SN2.
It was found that by the mechanism of SN2 the reaction (3) has no the energy barrier and should occur spontaneously (Fig. 6).
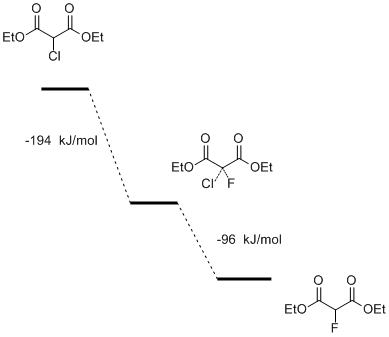
Fig.6. The energy profile of the substitution reaction of chlorine by fluorine in diethyl chloromalonate by the mechanism of SN2.
The influence of the solvent in this process does not introduce any fundamental changes. The free energy of formation of the transition state (reaction 3, stage I) in both polar and nonpolar solvents is the negative, and ΔG of the reaction increases with increasing solvent polarity (Fig. 7, Table 4).
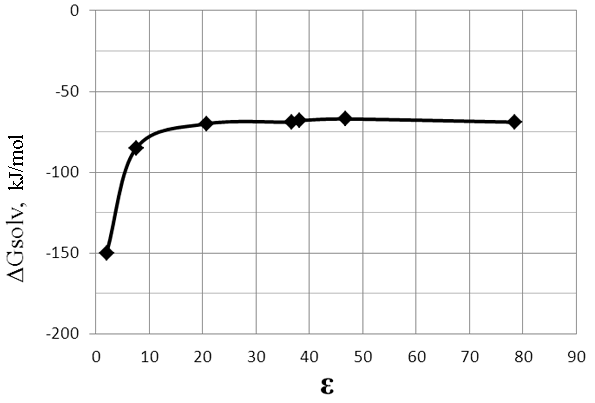
Fig.7.The dependence of ΔG of the reaction (3) (stage I) on the dielectric constant (ε) of solvent.
Table 4. The dependence of ΔG of the reaction (3) (stage I) on the dielectric constant (ε) of solvent.
|
Solvent |
ε |
ΔGsolv, kJ / mol |
|
Cyclohexane |
2,02 |
-150 |
|
Tetrahydrofuran |
7,58 |
-85 |
|
Acetone |
20,70 |
-70 |
|
Acetonitrile |
36,64 |
-69 |
|
Nitromethane |
38,20 |
-68 |
|
Dimethylsulfoxide |
46,70 |
-67 |
|
Н2О |
78,39 |
-69 |
The stage II of the reaction (3) is also thermodynamically very biased towards the formation of products, Δ G of the reaction decreases with increasing polarity of the medium (Fig. 8, Table 5).
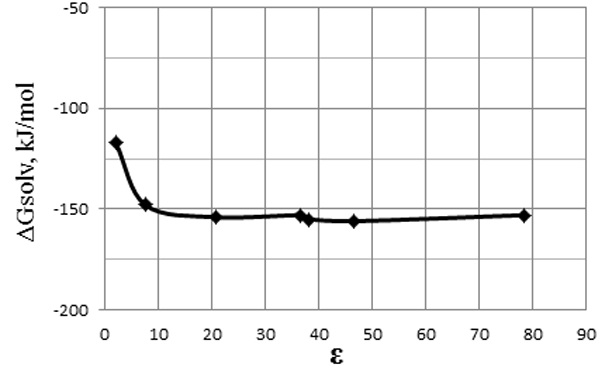
Fig.8. The dependence of ΔG of the reaction (3) (stage II) on the dielectric constant (ε) of solvent.
Table 5. The dependence of ΔG of the reaction (3) (stage II) on the dielectric constant (ε) of solvent.
|
Solvent |
ε |
ΔGsolv, kJ / mol |
|
Cyclohexane |
2,02 |
-117 |
|
Tetrahydrofuran |
7,58 |
-148 |
|
Acetone |
20,70 |
-154 |
|
Acetonitrile |
36,64 |
-153 |
|
Nitromethane |
38,20 |
-155 |
|
Dimethylsulfoxide |
46,70 |
-156 |
|
Н2О |
78,39 |
-153 |
Such results are contrary to the experimental data [11], where the substitution of chlorine by fluorine occurred only when heated to 150 °C.
However the reaction of nucleophilic substitution by the mechanism of SN2 depends not only on the concentration of the substrate, but also on the chemical potential of nucleophile in the solution. In this connection we have calculated the energy barrier of the nucleophilic substitution of chlorine by fluorine in diethyl chloromalonate taking into account the energies of dissociation of potassium fluoride and potassium chloride (Figure 9).
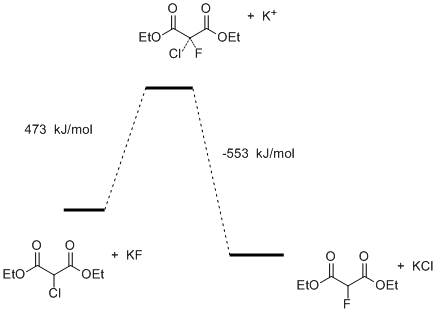
Fig.9. The energy profile of the substitution reaction of chlorine by fluorine in diethyl chloromalonate by the mechanism of SN2 in the gas phase taking into account the dissociation of potassium salts.
These calculations showed that the dissociation energy of potassium fluoride is high enough and the energy profile of the reaction takes other character (Fig. 9). The high energy barrier impedes the course of the reaction under normal conditions. This result is in agreement with the experimental data well. In addition, we have performed the thermodynamic calculations for this reaction in a solvent. It was found that the energy barrier of the reaction greatly reduced, approaching zero, with increasing polarity of the medium (Fig. 10, Table 6).
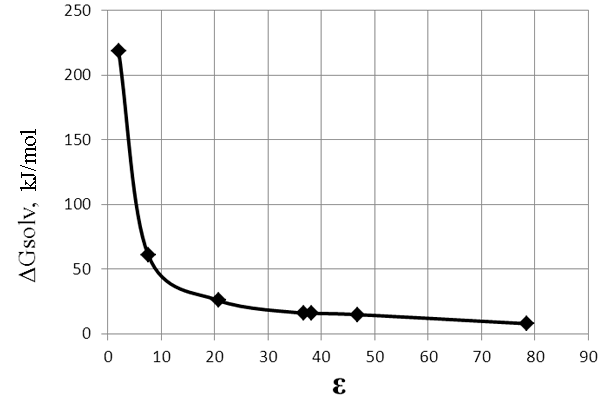
Fig.10. The dependence of ΔG of the reaction (3) (stage II) on the dielectric constant (ε) of solvent taking into account the dissociation of potassium salts.
Table 6. The dependence of ΔG of the reaction (3) (stage II) on the dielectric constant (ε) of solvent taking into account the dissociation of potassium fluoride.
|
Solvent |
ε |
ΔGsolv, kJ / mol |
|
Cyclohexane |
2,02 |
219 |
|
Tetrahydrofuran |
7,58 |
61 |
|
Acetone |
20,70 |
26 |
|
Acetonitrile |
36,64 |
16 |
|
Nitromethane |
38,20 |
16 |
|
Dimethylsulfoxide |
46,70 |
15 |
|
Н2О |
78,39 |
8 |
The Fig. 10 and Table 6 show that the energy barrier of the reaction is largely dependent on the dissociation of potassium salts. Conducting of the reaction in polar media allows to carry out the reaction under milder conditions by reducing the dissociation energy of KF and increasing the concentration of the fluoride anion.
To increase the concentration of the fluoride anion is possible and by increasing temperatures. This effect is observed experimentally when the substitution reaction of chlorine by fluorine in diethyl chloromalonate stopped at low temperatures [11]. Obviously, this temperature was insufficient for the dissociation of potassium fluoride and the necessary concentration of fluoride ion was not achieved.
The research was supported with financial support of the Ministry of Education and Science of Russian Federation within realization Federal Programs «Scientific and scientific- pedagogical shots of innovative Russia» for 2009-2013 years» and «Researches and development in the priority directions of development of a scientific and technological complex of Russia for 2007-2013 years».
References
1. Ishikawa N., Takaoka A., Ibrahim K. M. // J. Fluorine Chem. – 1984. – Vol. 25. –Р. 203.
2. Devadas B., Kishore N. S., Adams S. P., Gordon J. I. // Bioorg. Med. Chem. Lett. – 1993. – Vol. 3. – P. 779.
3. Hutchinson J., Sanford G., Vaughan J. F. S. // Tetrahedron. – 1998. – Vol. 54. – P. 2867.
4. Gryszkiewicz-Trochimowski E., Sporzynski A., Wnuk J. // Rec. Trav. Chim. – 1947. – Vol. 66. – P. 424.
5. Pattison F.L.M. et al. // Can. J. Chem. – 1965. – Vol. 43. – P. 1700.
6. Chambers R. D., Hutchinson J. // – 1998. – Vol. 92. – № 1. – P. 45.
7. Hiller A., Patt J. T., Steinbach J. // J. Organometallic Chem. – 2006. – Vol. 691. – №. 18. – P3737.
8. Machleidt H. // Justus LiebigsAnnalen der Chemie. – 1964. – Vol. 676. – P. 66.
9. Razrabotka novogo metoda sinteza diehtilovogo ehfira 2-ftormalonovoj kisloty: Otchet o NIR (promezhutoch.) / TGU; Rukovoditel' N. F. Mingalimov.GR 01201177736; Inv. 603063. Tomsk, 2011. 49 s.
10. Sintezy ftororganicheskih soedinenij / Pod red. I. L. Knunyanca, G. G. Yakobsona. M.: Khimiya, 1973. 312 s.
11 O.B. Sambueva, V.A.Yanovsky, N.F.Mingalimov, A. N.Tretyakov, O.S.Andrienko, V.I.Sachkov.Research of the reaction of diethyl chloromalonate with potassium fluoride in aprotonic solvents//Fluorine notes, 2012, № 3(82), URL: http://notes.fluorine1.ru/public/2012/3_2012/letters/rusletter4.html (дата обращения 26.06.2012)
Recommended for publication by Prof. S. Igoumnov
Fluorine Notes, 2012, 83, 7-8
