Received: March, 2012
Fluorine Notes, 2012, 83, 9-10
Derivatives Of 5,5,6-Trifluoro-7-oxabicyclo [2.2.1]hept-2-en-6-exo-carboxylic acid
K. N. Gerasimov, A. F. Eleev, V. S. Kuzmin, S. S. Khochlov
2 Federal State Unitary Entity State Scientific – Research Jnstitute of Organic Chemistry
and Technology, 111024, Moscow, 23 Shosse Entusiastov,
e-mail: dir@gosniiokht.ru
Abstract: Using method of X-ray structural analysis it has been found, that in the reaction of [2+4]-cycloattachment of perfluoroacrylonitrile to furan the exo-isomer of 5,5,6-trifluoro-7-oxa-bicyclo[2.2.1]hept-2-en-6-carboxylic acid was the main product. Derivatives based on it have been synthesized: acid, acid potassium salt, methyl ether of acid, 2,2-dichloro-5,5,6-trifluoro-trifluoro-7-oxa-bicyclo-[2.2.1]heptan-6-carboxylic acid.
Keywords: Perfluoroacrylonitrile, furan, [2+4]-cycloattachment, derivatives of 5,5,6-trifluoro-7-oxabicyclo[2,2,1]hept-2-en-6-carboxylic acid, X-ray structural analysis, NMR-spectroscopy.
In search for potential inhibitors of shikimate kinase, of which shikimic acid is a substrate, a synthesis of 5,5,8-trifluoro-7-oxa-bicyclo[2.2.1]hept-2-en-6-exo-carboxylic acid derivatives has been conducted in order to study their antituberculous (TB resistance) activity. Using method of NMR-spectrometry it has been found, that two isomers 1 and 2 are the products of perfluoroacrylonitrile to furan [2+4]-cycloattachment at ratio equal to 65 : 35. The reaction can be described as follows (using the following scheme listed below):
After isolating the isomers (preparative GLC) in the indivial state using method of PCA it has been stated, that exo-isomer is the main product 1.
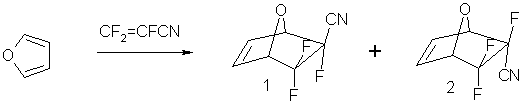
The structure of the molecule 1 has been stated using the method of X-ray structural analysis. Molecule overall layout incl. numbering atoms in it is displayed at Pic.1.
Pic.1. Molecule 1 Structure and Atoms Numbering
Bond lengths and valence angle are listed in Table 1. All of them show ordinary figures for bicyclic system and point out its asymmetry, which probably is caused by electronic effects created by different substituents at atoms C (1) and C (2). The length of double bond С(4)-С(5) coincides with its standard value of 1,33 Å [1].
The fragment of С(3)-С(4)-С(5)-С(6) is virtually flat; the torsion angle is equal to 0,6°. Atoms’ Mean-square deviations from the surface amount to 0.002, -0.003, 0.003, -0.002 Å, respectively. The oxygen atom deviates from this surface for 0,699 Å, that is equal to dihedral angle of С(3)-O(1)-С(6) fragment bend (flange) from mentioned above surface for 132,9°.
The second cycle of bicyclic system is also folded along the line С(3)…С(6), but to a greater extent: the angle between surfaces of С(6)-С(1)-С(2)-С(3) and С(3)-О(1)-С(6) is equal to 116,0°. It is interesting, that in this cycle the fragment of С(3)-С(2)-С(1)-С(6) is virtually flat with small exits of atoms from the medium surface of 0.003, -0.004, 0.004, -0.003 Å, corresponding to the value of torsion angle of 0,1°. The bigger excessive bend of this cycle is caused obviously by the fact, that sp3-hybrydization is inherent to all the carbon atoms it contains.
The geometry of nitrile substituent is a usual linear one with an angle at atom C (7) equal to 176,9°.
Table 1. Bonds Lengths (Е) And Valency Angles (degrees) In The Molecule 1, standard deviations are bracketed.
|
С(1)-С(2) |
1,574(4) |
С(6)-O(1) |
1,449(3) |
|
С(2)-С(3) |
1,575(4) |
C(1)-F(1) |
1,358(3) |
|
С(3)-С(4) |
1,519(4) |
C(1)-F(2) |
1,354(3) |
|
С(4)-С(5) |
1,330(4) |
C(2)-F(3) |
1,382(3) |
|
С(5)-С(6) |
1,508(4) |
C(2)-C(7) |
1,473(4) |
|
С(6)-С(1) |
1,543(4) |
C(7)-N(1) |
1,147(4) |
|
С(3)-O(1) |
1,437(3) |
C(3)-C(2)-C(7) |
111,1(2) |
|
C(3)-O(1)-C(6) |
97,2(2) |
F(3)-C(2)-C(7) |
108,6(2) |
|
C(6)-C(1)-C(2) |
102,0(2) |
C(2)-C(3)-O(1) |
98,2(2) |
|
C(6)-C(1)-F(1) |
114,3(2) |
C(4)-C(3)-O(1) |
103,1(2) |
|
C(6)-C(1)-F(2) |
112,1(2) |
C(1)-C(6)-C(5) |
107,0(2) |
|
C(2)-C(1)-F(1) |
110,7(2) |
C(1)-C(6)-O(1) |
98,1(2) |
|
C(2)-C(1)-F(2) |
112,1(2) |
C(5)-C(6)-O(1) |
103,0(2) |
|
F(1)-C(1)-F(2) |
106,7(2) |
C(3)-C(4)-C(5) |
105,8(3) |
|
C(1)-C(2)-C(3) |
99,9(2) |
C(4)-C(5)-C(6) |
106,2(2) |
|
C(1)-C(2)-F(3) |
110,5(2) |
C(3)-C(2)-C(7) |
111,1(2) |
|
C(1)-C(2)-C(7) |
112,8(2) |
C(2)-C(7)-N(1) |
176,9(3) |
|
C(3)-C(2)-F(3) |
113,8(2) |
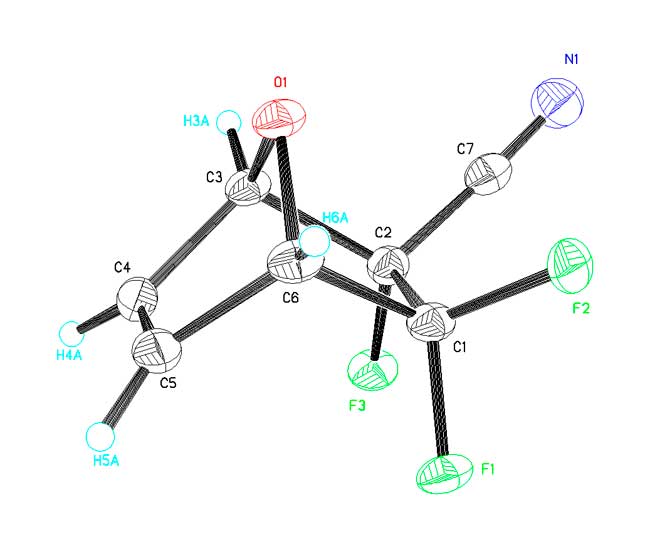
As the compound is distinguished by high volatility it was interested to analyze its crystal package. The fragment of crystal package is displayed at Pic. 2. There are no short intermolecular contacts which could be interpreted as weak specific interactions, for example, between nitrogen atom of nitrile group and rather acidic atoms of hydrogen at atoms of С(3) and С(6). Such weak interactions of electrostatic nature and which are being discussed in the works [2 – 5] can control (to a great extent) the structure of a crystal itself. All the intermolecular contacts in the crystal package under consideration correspond to Van der Vaals interactions. The absence of specific interactions in the crystal of present compound explains its high volatility.
Pic. 2. Fragment of Crystal Package.
The isolation of exo-isomer in the individual form allowed carrying out the synthesis of different derivatives of 5,5,5-trifluoro-7-oxabicyclo[2.2.1]hept-2-en-6-exo-carboxylic acid based on it. Under the conditions of alkali hydrolysis the potassium salt 3 has been obtained with the yield of 83%. During processing of salt 3 using hydrochloric acid with further isolating out of aqueous solution an acid 4 has been obtained with the yield of 93%. In the reaction of potassium 3 with dimethylsulphate a methyl ester of 5,5,5-trifluoro-7-oxabicyclo[2.2.1]hept-2-en-6-exo-carboxylic acid 5 has been synthesized with the yield of 71%. While chlorinating salt 3 in the medium of hydrochloric acid the corresponding product of chlorine attachment by/acc the bicyclohepten’s double bond – corresponding bicycloheptan 6 has been obtained with the yield of 25%. The synthesis of mentioned compounds is illustrated by the following scheme:
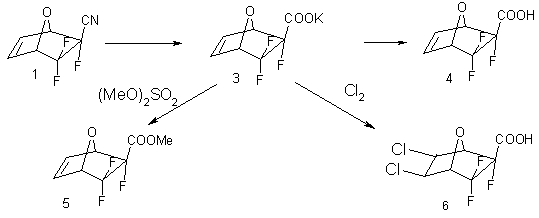
The structure of compounds 1-6 has been proved by the data of 1H and 19F-NMR, IR and mass-spectroscopy.
Soluble in water compounds 3, 4 and 6 are characterized by moderate toxicity; LD50 for white mice at intramuscular application amount to >1500 mg/kg, 300 mg/kg and 1600 mg/kg, respectively.
Experimental Part
X-ray Structural Experiment
The experimental set of intensities for PCA has been obtained at automatic diffractometer Bruker SMART-CCD (Мо-radiation, graphite monochromator, scanning) in the stream of colled nitrogen. Due to the volatility of compound 1 the monocrystal covered with perfluorinated oil was used. Crystallographic parameters and characteristics of x-ray structural experiment are listed in Table 2.
Editing of experimental reflections was conducted using software package Bruker SAINT [6].
The structure is deciphered using direct methods and made more accurate using method of the smallest squares in a full matrix anisotropic approximation along F2. Hydrogen atoms have been discovered from the differential Furrie synthesis. Their adjusting has been carried out in the isotropic approximation. All of the calculations have been carried out using SHELXTL-Plus software package [7].
The coordinates of non-hydrogen atoms and their isotropic equivalent temperature parameters are listed in Table 3.
Table 2. Parameters Of Crystal And X-ray Structural Experiment
|
Compound 1 |
Nitrile 5,5,6-trifluoro-7-oxabicyclo[2.2.1]hept-2-en-6-exo-carboxylic acid |
|
Molecular formula |
C7H4NF3O |
|
Molar Mass (kg/kmole) |
175,11 |
|
Syngony |
Triclinic |
|
Spatial Group |
P 1 |
|
6,596(1) |
|
7,188(1) |
|
7,501(1) |
|
91,69(3) |
|
91,54(3) |
|
108,68(3) |
|
336,5(1) |
|
2 |
|
ρ(cal.), g/cm3 |
1,728 |
|
F(000) |
176 |
|
μ(MoKα), mm-1 |
0,172 |
|
Crystal Size, mm |
0,28 × 0,32 × 0,16 |
|
Temperature, °K |
120,0(2) |
|
Radiation, Å |
Mo Kα(0,71073) |
|
Type/scanning field along θ, (°) |
ω / 2,72 – 26,98 |
|
Intervals of Reflection Indices |
-4 ≤ h ≤ 8, -9 ≤ k ≤6, -9 ≤ l ≤ 8 |
|
Reflections being recorded and measured |
1695 |
|
Independent Reflections |
1305 [R(int) = 0.0124] |
|
Reflections с I>2σ(I) |
1305 |
|
Variables of Accuracy |
126 |
|
R -factors acc to I>2σ(I) |
R1 = 0,0531; wR2 = 0,1875 |
|
By all reflections |
R1 = 0,0622; wR2 = 0,1962 |
|
Quality factor acc to F2 |
1,080 |
|
Residual Electron Density, min/max, e/Å3 |
|
Table 3. Coordinates Of Non-hydrogen Atoms (x104) And Isotropic Equivalent Temperature Parameters (U2 x 103). Standard deviations are bracketed.
|
Atom |
x |
Y |
z |
U(eq) |
|
F(1) |
3959(3) |
2167(2) |
5934(2) |
22(1) |
|
F(2) |
5312(2) |
4096(2) |
8257(2) |
22(1) |
|
F(3) |
1775(3) |
4339(2) |
5006(2) |
21(1) |
|
O(1) |
1021(3) |
3037(3) |
9475(2) |
19(1) |
|
N(1) |
3936(4) |
8124(4) |
7887(4) |
29(1) |
|
C(1) |
3444(4) |
3057(4) |
7395(3) |
17(1) |
|
C(2) |
2126(4) |
4440(4) |
6836(4) |
17(1) |
|
C(3) |
36(4) |
3510(4) |
7896(3) |
17(1) |
|
C(4) |
-1103(5) |
1508(4) |
7002(4) |
20(1) |
|
C(5) |
40(5) |
363(4) |
7469(4) |
20(1) |
|
C(6) |
1896(5) |
1638(4) |
8632(4) |
18(1) |
|
C(7) |
3194(4) |
6508(4) |
7415(4) |
20(1) |
Spectral Data
NMR 1Н and 19F spectra have been obtained at Spectrometer AVANCE-400 of “Bruker” company with operational frequencies of 400 (1Н) and 376,5 (19F) MHz respectively. Shooting range were performed at room temperature in CDCl3 (сompounds 1-2), D2O (3) and CD3CN (4-6). Chemical shifts in NMR spectra are shown in the δ-scale regarding inner standard: ТМS (1H) and CFCl3 (19F).
IR-spectra were recoreded using spectrometer Perkin-Elmer PE-286 in the solutions in the thin layer at KBr.
Mass-spectra were recorded using mass-spectrometer VARIAN MAT CH-6 equipped with device of direct input, 70 эВ.
5,5,6-Trifluoro-6-cyane-7-oxabicyclo[2.2.1]hept-2-ens (1 and 2). The solution of 45,0 g (0,417 mole) of perfluoroacrylonitrile and of 16,0 g (0,235 mole) of furan in the 50 ml of diethyl ether was being heated in the sealed vial at 95-100°С for 21 h. The further distillation in vacuum has produced 12,8 g (0,73 mole, 31%) of 1 and 2 compounds mixture in the form of viscous liquid with b.p. of 95 °С at 20 mm mercury column. Found, %: С 48,15; Н 2,46; N 7,93. C7H4NF3O. Calculated, %: С 48,01; Н 2,29; N 8,00. Using method of preparative GLC two isomers 1 and 2 have been isolated. The conditions of separation: chromatographer Perkin-Elmer F21; column–chromaton, NAV DMS 5% XE-60, the length of column is 5m; evaporator’s and branched and connecting tubing temperature is 230 °С; thermostat temperature is 129 °С.
The m.p. of 5,5,6-trifluoro-6-exo-cyane-7-oxabicyclo-[2.2.1]-hept-2-en (1) is 48 °С. NMR 19F spectrum (50% CDCl3): 157,3 s (6-F, 1F); 107,0 m (5-F, 2F). NMR 1Н spectrum (50% CDCl3):5,23 s (4-Н, 1Н); 5,55 s (1-Н, 1Н); 6,68 s (2-Н, 3-Н, 2Н).
IR-spectrum (in CCl4), ν, cm-1 (%, T):3029 (95), 2253 (96), 1659 (98), 1579 (96), 1311 (50), 1290 (56), 1274 (55), 1240 (85), 1211 (98), 1186 (28), 1123 (41), 1108 (24), 1057 (86), 1040 (81), 992 (35), 973 (71), 917 (56), 827 (89), 809 (100), 777 (67), 750 (21), 742 (100), 738 (90), 733 (95), 685 (84), 661 (81), 620 (95), 607 (64), 520 (97), 481 (96), 465 (95), 433 (98), 419 (97).
The m.p. of 5,5,6-trifluoro-6-endo-cyane-7-oxabicyclo-[2.2.1]–hept-2-en (2) is 42 °С. NMR 19F spectrum (50% CDCl3):177,0 s (6-F, 1F); 114,8 d (5-Fexo, J= 225 Hz; 1F); 101,2 d (5-Fendo, J= 225 Hz; 1F). NMR 1Н spectrum (50% CDCl3):4,77 s (4-Н, 1Н); 5,10 d (1-Н, J= 6,3 Hz, 1Н); 6,50 br.s (2-Н, 3-Н, 2Н).
IR-spectrum (in CCl4), ν, cm-1 (%, T):3164 (53), 3110 (47), 3044 (53), 2959 (49), 2878 (50), 2439 (59), 2253 (55), 1973 (59), 1903 (59), 1660 (57), 1580 (48), 1461 (56), 1413 (55), 1385 (55), 1311 (29), 1038 (25), 987 (19), 971 (29), 940 (39), 919 (24), 831 (41), 793 (23), 741 (18), 686 (45), 674 (30), 608 (31), 595 (50), 531 (50), 496 (52), 412 (22).
In the mass-spectra of both isomers a value m/e (%): 175 (M+, 100) has been recorded.
Potassium salt of 5,5,6-trifluoro-7-oxa-bicyclo-[2.2.1]hept-2-en-6-exo-carboxylic acid (3). The mixture of 4,5 g (25,7 mmole) of bicyclohepten (1) in 10ml of dioxane and 2,9 g (51 mmole) of potassium hydroxide in the 5ml of water has been being heated while stirring up to 110-120 °С and kept at that temperature for 3 hours. Further, the reaction mass has been evaporated in vacuum, the residue has been washed through with dioxane. We have obtained 5,0 g (83%) of colorless crystallic compound (3), the decomposition temperature with at about 200 °С and well soluble in water (no less than 30%). Found, %: С 36,55; Н 1,95. C7H4F3О3К. Calculated, %: С 36,05; Н 1,72.
NMR 19F spectrum (30% D2О):151,5 s (6-F, 1F); 111,0 d.d (5-Fexo, J= 240 Hz; J= 4,5 Hz; 1F); 107,0 d (5-Fendo, J= 240 Hz; 1F). NMR 1Н spectrum (30% D2О):5,30 s (1-Н, 1Н); 5,17 d (4-Н, J= 4,5 Hz, 1Н); 6,83 br.s (2-Н, 3-Н, 2Н).
5,5,6-Trifluoro-7-oxabicyclo[2.2.1]hept-2-en-6-exo-carboxylic acid (4). To the suspension of 0,90 g (3,86 mmole) of salt (3) in the 20 ml of chloroform 7 ml of concentrated hydrochloric acid have been added, the organic layer was separated, aqueous layer has been extracted 5 ml of chloroform?. The unified solutions in chloroform were evaporated, using re-crystallization of residue 0,71 g (93%) of colorless crystallic compound (4) with melting point of 62 °С were obtained. Found, %: С 43,51; Н 2,89. C7H5F3О3. Calculated, %: С 43,30; Н 2,58. Mass-spectrum, m/e (%): 194 (M+, 100).
NMR 19F spectrum (25% in CD3CN):158,2 s (6-F, 1F); 111,3 d.d (5-Fexo, J= 225 Hz; J= 4,5 Hz; 1F); 106,7 d (5-Fendo, J= 225 Hz; 1F). NMR 1Н spectrum (25% in CD3CN):5,13 s (1-Н, 1Н); 4,80 d (4-Н, J= 4,5 Hz, 1Н); 6,47 br.s (2-Н, 3-Н, 2Н); 7,50 s (СООН, 1Н).
Methyl Ester of 5,5,6-trifluoro-7-oxabicyclo-[2.2.1]hept-2-en-6-exo-carboxylic acid (5). The mixture containing 3,10 g (13,3 mmole) of salt (3) and 1,68 g (13,3 mmole) of dimethylsulphate in 20ml of absolute dioxane have been being stirred for 3h at 140-150 °С. Further the mixture was filtered, and the filtrate itself had been distilled in vacuum. We received 1,25g (about 40%) of colorless compound (5) with m.p. of about 15 °С and b.p. of 70-71 °С at 1 mm of mercury. Found, %: С 46,52; Н 2,99. C8H7F3О3. Calculated, %: С 46,15; Н 3,37. Mass-spectrum, m/e (%): 208 (M+, 100).
NMR 19F spectrum ((40% in CDCl3):161,5 s (6-F, 1F); 112,2 d.d (5-Fexo, J= 227 Hz; J= 4,5 Hz; 1F); 107,5 d (5-Fendo, J= 227 Hz; 1F). NMR 1Н spectrum (25% in CD3CN):5,15 s (1-Н, 1Н); 4,77 d (4-Н, J= 4,5 , Hz 1Н); 6,51 b.s (2-Н, 3-Н, 2Н); 3,75 s (ОСН3, 3Н).
2,3-Dichlorine-5,5,6-trifluorine-7-oxa-bicyclo[2.2.1]-hept-2-en-6-carboxylic acid (6). Via the solution of 1,80 g (7,73 mmole) of salt (3) in the 12 ml of 10% hydrochloric acid at intensive stirring at 75-80 °С app. 5 l of chlorine were bubbled. After that the mixture has been evaporated in vacuum, and by re-crystallizing of the residue from chloroform a 0,50 g (25%) of colorless crystallic compound (6) has been obtained with m.p of 92 °С. Found, %: С 31,02; Н 2,08; Cl 26,01. C7H5Cl2F3О3. Calculated, %: С 31,82; Н 1,89; Cl 26,59. Mass-spectrum, m/e (%): 265 (M+, 100).
NMR 1Н Spectrum (25% in CD3CN):4,90-4,98 broad signal (1-Н, 4-Н, 2-Н, 3-Н, 4Н); 6,23 s (СООН, 1Н).
Referencies
- F. H. Allen, O. Kennard, D. G. Warson, L. Brammer, A.G. Orpen, Robin Taylor, J. Chem. Soc. Perkin Trans, II, 1987, S1-S19
- G.A. Jeffrey, W. Saenger, “Hydrogen bonding in Biological structures”, Springer, Berlin, 1991.
- J. A. R. P. Sarma, G. R. Desiraju, Acc. Chem., Res., 1986, 19, 222.
- G. R. Desiraju, J. Chem Soc. Chem. Commun., 1989, 179.
- V.R. Pedireddi, G.R Desiraju, . Chem Soc. Chem. Commun., 1992, 988.
- SAINT. Version 6.02A. Bruker AXS Inc. Madison Wisconsin, USA, 2001
- SHELXTL-Plus. Release 5.10, Bruker AXS Inc., Madison, Wisconsin, USA, 1997.
Recommended for publication by Prof. Alexander F. Eleev
Fluorine Notes, 2012, 83, 9-10
