Received: May, 2012
Fluorine Notes, 2012, 83, 5-6
ABOUT FLUORINE-CONTAINING SURFACE-ACTIVE SUBSTANCES CAPABLE TO MODIFY POLYMER FABRICS' FIBERS
A.A. Ageev1, I.V. Aksenova1, V.A. Volkov1, A.F. Eleev2
1 Moscow State Textile University named after A.N. Kosygin, State Educational Establishment
of Higher Professional Education, 119071, Moscow, 1 Malaya Kaluzhskaya Street,
e-mail: micella@inbox.ru
2 Federal State Unitary Entity State Scientific – Research Jnstitute of Organic Chemistry
and Technology, 111024, Moscow, 23 Shosse Entusiastov,
e-mail: dir@gosniiokht.ru
Abstract: Surface modification of textile fibers allows to get new materials with programmed
properties, in particular, water- and oil-repellent ones. Fluorine-containing substances proved to
be the most effective for achieving this goal. They can form molecular coatings of a regular structure
on the surface of polymer fibers when they form nano-size rough coatings in combination with water-soluable
complexes which in their turn reinforce a modifying affect.
The paper presents the results of
the research on polymer fibers surface modification with the aim to receive hydrophobic and oilphobic
textile materials for protection clothes excluding human contact with harmful substances. An original
technology for fibers' modification aimed at producing textile materials with high protection properties
has been worked out on the results of the research on contacts between fluorine-containing substances
and textile fibers surfaces.
Keywords: Fluorine, fluorine-containing substances, surface-active substances (SAS), polymer fibers, textile materials, hydrophobia, oil-repellent, nano-roughness, polymer coating, molecular layer-on-layer cover, adsorption.
Introduction
The production of protection clothes capable to withstand the penetration of harmful substances through textiles thus contacting a human body and negatively affecting his/her health is believed to be one of the most important tasks in protecting people from the negative influence of the environment’s. A modification of textiles’ fibers leading to their inability to be soaked with harmful substances, i.e. oils and oil-like liquids, is being carried out to achieve this goal.
Fluorine-containing compounds are used to provide fibers with oil-repellent properties. A critical surface tension in a cotton fabric can achieve its minimal size of 7 mJoule/m2 if it is modified with per fluorine lauric acid (12 carbon atoms) and makes only 10 mJoule/m2 if per fluorine butyric acid is used. To achieve a stable quality in the oil-repellent coating they traditionally use modifying polymers which contain in their angle chains fluorocarbon radicals of the following type F3C-(CF2)n-R-, where n≥ 6; but to fasten fibers on the surface it’s necessary to have in the main chain the groups capable to interact with reactionable groups of fibers’ surface. Actually, to achieve the desired effect of oilphobiability in textile fabric (when the surface’s outer specific activity is ~ 4 m2/gr) it’s necessary to put 0,6…0,7 % from the fabric mass of polymer in whose side chains there are fluorocarbon chains containing not less than 7 atoms of carbon, i.e. heptaperfluoroacrylate [1].
In this case a textile material modification is done due to the adhesion of the polymer’s molecules in the form of a coating on the fabric fibers. The simplicity of such a modification of fabric leads to from a grave shortcoming, i.e. a comparatively low resistance in modifying substances on the surface of fibers when the fabrics are to undergo dry cleaning or washing. Besides, if a modification is done out of polymer’s dispersion – latexes, it comes out that a part of fibers surface becomes closed (covered) by the molecules of latex’s hydrofile SAS-stabilizes and it greatly decreases the fluorine-containing substance’s modifying impact. Thus, an e emulsifier’s negative influence upon the fluoropolymer’s modifying function appears to be a shortcoming when a fabric s modified by a polymer in the form of a dispersion (latex). In this case mach of fibers surface will be covered by hydrofile groups which reduce a modification efficiency. For instance, according to some data [2] one can reach a surface tension in modified fibers at the level of only 19-24 mJoule/m2 if latex with 3-5% emulsifier is used, whereas it can make ~ 10 mJoule/m2 if there is no emulsifier. The research work on a change in the orientation of stabilizer’s molecules in the adsorption layer on the surface of modified fibers among the films of modifying polymer was carried out by us with the use of polyvalent cations [3], fluorine-containing [4] or carbon cation SAS [5]. This type of additional processing (treatment), being irrational in the technology of getting textile materials, is absolutely real and possible in the modification of textile articles at chemical cleaning enterprises or in industrial laundries [1]. However, multi-staged processing of fabrics or cotton goods always requires extra labour and resources.
Thus, an urgent need to develop a modification process (method) excluding the modified surface fragmentation and combined with the lowest labour and resources expenses ensues. It’s possible if one uses latexes of fluorocarbon emulsifies for synthesis; but nowadays it’s unreal, or if one modifies fibers by the nano-technological method of putting molecules of fluorocarbon surface-active substances (FSAS) layer-on-layer onto the surface of fabrics’ fibers with their further strengthening there thus increasing their resistance to washings and chemical cleaning.
Fluorine-containing SAS to Modify Fibers
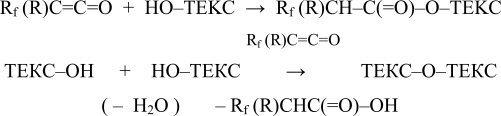
At present fluorine-containing surface active substances (SAS) are represented practically by all classes of organic compounds. They are mainly various derivatives from fluorine-containing acids; salts, including organic salts with a cation on the basic of the first, second, third and fourth nitrogen atom, ethers and amids as well as their various combinations. A chemical modification based on chemical links between surface active fluorine-containing fragments (marked as Rf hereinafter) and hydroxyl or aminogroups on the surface of textile’s fibers is possible if one uses such reaction-able fluorine-containing compounds as anhydrides and halogen anhydrides of acids, ketenes, isocyanates and other compounds of this type. A serious deficiency in the use of anhydrades and halogenanhydrides is believed to be a formation of chemically aggressive side-products of the reaction: acids or halogenhydrogen according to the scheme (for a modification with hydroxyl textile’s groups, marked as HO-TEKC)
![]()
where Х : Rf С(=O)O, F, Cl, Br, OSO2X etc.
A dehydrotation leading to a destruction of textile fibers seem to be a by-process of hydroxyl groups acyllation when such highly reaction-able reagents as fluorine-containing ketenes or isocyanates are used. For ketenes the process is described by the below given scheme:
A principally new variant of chemical modification of textile’s fibers is achieved with the implementation of surface-active fluorine-containing ketones. In comparison with anhydrides and halogenanhydrides of fluorine-containing acids corresponding fluorine-ketones ensure a chemical links between fluorine-containing surface-active fragments and textile fibers due to a construction of ketones according to the scheme:
![]()
A more “rigid” (“hard”) link between fluorine-containing surface – active fragments with textile (an acyllation of hydroxyl groups) appears in a “haloformed” decay (dissociation) of a newly formed ketal according to the scheme:
![]()
In this case phluorketones serve as extremely “soft” acyllating reagents. Contrary to acyllating reagents of acid’s anhydrides and halogenanhydrides type, the newly formed products of acyllation are not aggressive substances in case when fluororketones are ketones is characterized by a higher safety level of implementation aimed at the modification of textile.
The best studied fluororketon for the purpose of textile modification happens to be perfluoroundecan-3-on-2 sylfonylfluoride (PFSK-8) which is received in the reaction between chloranhydrade of perfluoropelargon acid and 1-hydrotetrafluoroethanesylphonyl-fluorid in the presence of triethylamin [6]:
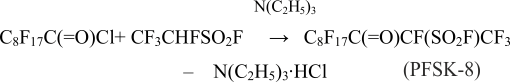
PFSK-8 water solutions show their surface-active properties due to a formation of geminal diol’s molecules in water [7-9]:
![]()
PFSK-8 fluororketon is one of the first samples of surface-active fluoroketones. A further possible expansion in the fluororketones’ types (assortment) will be done with the use of more available and ecologically safe ones. As a source of such compounds from the viewpoint of their availability, the most preferable are thought to be derivatives of hexafluoropropylene trimer oxide – fluoroanhydride perfluoro-2,5-dimethyl-3,6-dioxane acid:
![]()
This fluoroanhydride laid the basis for receiving fluororketones of three different structural types which can be used modify textile. An acyllation of malon acid’s ethers helps to the receive ketones of the first type and is described as follows [10]:
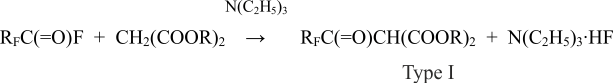
where RF = C3F7ОCF(CF3)CF2OCF(CF3) – ; R = CH3, C2H5
Fluoroketones of the second type are received in the acid hydrolysis of type I fluororketones. Applied to the quite definite Rf radical one receives corresponding methylperfluoroalkilketones by the following scheme [11]:
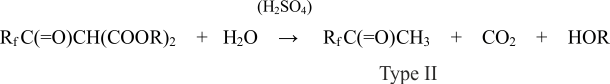
Fluoroketones of the third type are received in the reaction between type II fluoroketones and sulfuric anhydride by the following scheme [12]:
![]()
It’s worth mentioning that fluorine-containing ketosulfonic acids (type III), in their turn, are precursors of the forthcoming numbers of fluoroketones. Corresponding IIIa salts of various metals and ammonium can be received in the course of III type keton sulfonic acids’ neutralization or with the presence of cation on the basis of the primary, secondary, third or fourth nitrogen atom:
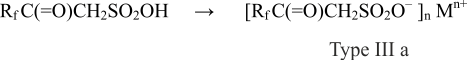
where M- cation of metal (n=1-3) or when n=1 nitrogencontaining cations NH4, NH3R, NH2R2, NHR3, NR4
In reaction with phosphorus pentachloride it’s possible to carry out a synthesis of corresponding type IIIb ketosulfochlorides which, in their turn, will be turned into corresponding IIIb type ethers and sulfonamide IIId
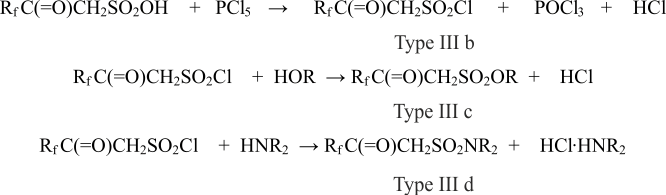
All the received fluoroketones will be tested and evaluated as textile modificators aimed at giving textiles hydro- and oilphobic properties. We plan to choose the most efficient and optimal in its purpose fluoroketon and to work out a special technological method of its production in accordance with the evaluated demands. It’s important to note that some of the analyzed purposeful ketones can find their practical implementation right now in everyday activities not only as modificators of fabrics. Indiev salt of IIIa type ketonesulfuric acid, when Rf = C3F7ОCF(CF3)CF2OCF(CF3), in particular, can be used as an alloying addition to motor oils [13].
Regularities of formation fluorine-containing substances' nano-layers
To achieve maximum efficiency a modification of fibers should be done so that fluorocarbon chains were located quite normally to their surface. The orientation can be reached with a formation of the modifying layer out of a non-polar environment, for instance, FSAS solutions in perchloroethylene. According to P.A. Rebinder’s rule of polar levelling (equilibrium) FSAS molecules’ orientation will take such a form when polar groups will be directed to the polar fragments of fiber’s surface and fluorocarbon chains will be directed into perchloroethylene, and after fabric’s drying out they’ll retain their orientation. If the layers’ formation is done out of water solutions the molecules’ orientation will become quite opposite, i.e. polar groups will appear outward and the surface will remain hydrophilic. With this in view, we’ve done the research on PFSK-8 adsorption out of perchloroethylene’s medium and defined the newly built layers’ modifying behavior.
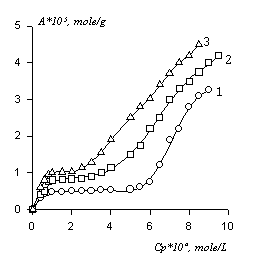
We’ve found out that PFSK-8 adsorption depends on fibers’ moisture and increases when the degree of their moisture grows. The example showing the dependence of PFSK-8 adsorption upon fibers’ moisture is given in figure 1, according to the data [14].
|
Fig.1. Isotherms of PFSK-8 adsorption on cotton at 293 K. A relative fabric's humidity, %: 1-0, 2-65, 3-81. |
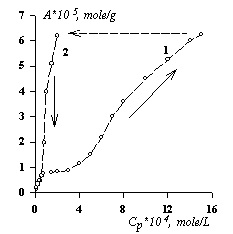
Fig.2. Isotherms of adsorption - desorption of PFSK-8 on a cotton fabric made of PCE at 293 K and fibers' relative humidity 65%. |
A calculation of adsorption layer’s parameters showed that an adsorption layer composed of 7 – 8 monomolecular layers happens to be formed on fibers’ surface. The formation of such a layer becomes possible only when SAS molecules are able to diffuse into fibers’ depth (inside).
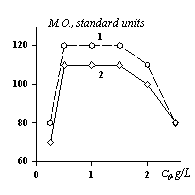
Fig.3. The influence of PFSK-8 solutions' initial concentration on modified fabrcs' properties: 1 – cotton, 2 – wool.
Actually, a research of a reversibility process of PFSK-8 adsorption on fibers helped to find out an adsorption hysteresis [14] reflected in fig.2. It shows the imbalance of the absorption process of FSAS on fabric’s cotton fibers. This fact may have a positive meaning in case if no chemical fixation of the modification is done as the SAS which is located inside fibers will compensate the losses of the modifier when articles (wares) undergo chemical cleaning. A formation of a SAS monomolecular layer will be quite enough if it’s necessary «to sew» modifying molecules to reaction able groups of fibers. If the amount of modifier prevails the quantity necessary for the monolayer it’ll lead to both an irrational SAS expenditure and a decrease of the modification effect [1]. For example, fig. 3 shows a dependence of cotton fibers surface modification efficiency upon the concentration of PFSK-8 solutions.
One can see that that oil repulsion goes through its maximum corresponding to a monolayer’s formation. An extra quantity of an adsorped substance is likely to change a molecules’ orientation in the second adsorption layer and a fall of modification efficiency ensues.
A creation of a uniform hydrophobic layer on a fibers’ plane surface out of molecules of fluorine-containing substance by the method of molecular-layer-on-layer putting enables to reach the size of fabrics’ angle-corners of watering θ=120°. A more efficient protection function can be achieved if to build a rough hydrophobic surface in the form of columns. In this case a so-called «lotus effect» is likely to appear and to increase the angle-corner of watering up to superhydrophobic indices, i.e. Θ ~ 160°. This phenomenon may be described by Cassy – Buxter’s equation:
cos Θн = fr cos Θ0 + f - 1, (1)
where cos ΘH, os Θ0 is an observed angle-corner of watering in both a real rough fabric and a plane; r - coefficient of roughness. which is evaluated in relation to the surface of a real body to that of an absolutely smooth one; f – is a projection share of a surface moisted with liquid. A picture of such a layer is given in fig.4.

Fig. 4. A picture of a rough layer in the form of unmoisted columns.
A drop of liquid, falling down onto such a surface, doesn’t penetrate into clearances (slots) among the columns but contacts only their tops. Hence, the contact area is less than the projection of the drop’s basic, and the effort leading to the drop’s spread essentially decreases too.
This kind of surface happened to be made with a consecutive putting a two-layer coating, the first layer being formed from macromolecules and the second one from SAS molecules. In our research we used chitozan acetate as a polymer able to remain stable on fibers’ surface while an interpolymer complex was being formed. The second layer was formed out of fluorine-containing SAS molecules able to interact with the remaining ammonium groups (phtoron 301) or with hydroxyl chitozan groups (PFSK-8) chemically.
Fig.5 gives a microphotography of a modified surface made with ion filled emission microscope (IEM). On the surface one can see polymer columns which don’t become moisted either covered with the second layer, i.e. fluorine-containing SAS.
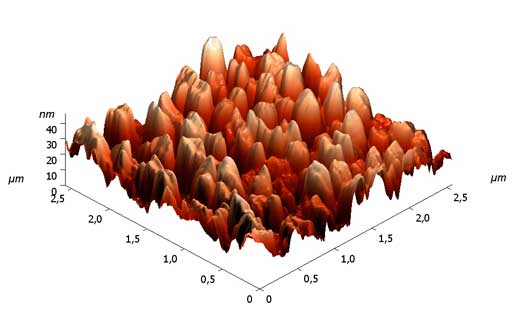
Fig.5. A microphotography of a mixed (cotton/polyether) fabric's surface modified with a consecutive layer-on-layer putting of chitozan and PFSK-8.
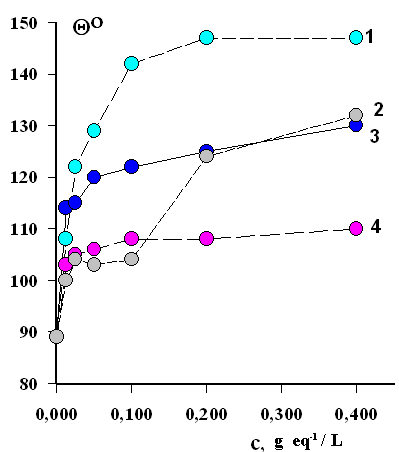
Fig.6 shows how the angle-corner of watering in a modified cotton fabric, moisted with water or silicone oil, depends upon the concentration of the modifying solution.
|
Fig.6. An impact of interpolymer (chitozan – phtoron 301) comlexes' concentration on the angle-corner of watering of modified fabrics with water (1,3) and paraffin oil (2,4). Multiplicity of fabrics' processing: once (2,4), twice (1,3). |
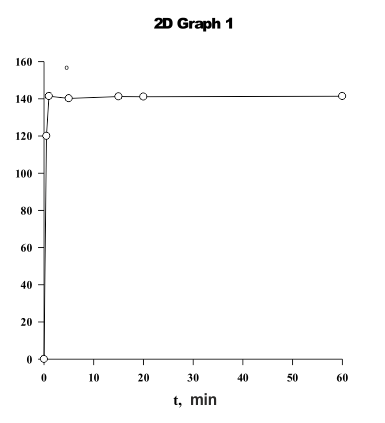
Fig.7. The impact of processing time when PFSK-8 solution is used for a mixed fabric after being covered with a chitozan coating. It's clearly seen that a protection layer is being formed during a short time period, i.e. 1 minute. |
All the above given pictures show that a high modifying effect in fabrics’ protection from being moisted with water and oil can be achieved in the process of building a modifying layer by the method of creation a molecular multilayered coating.
References
- Ageev A.A., Volkov V.A., Poverhnostnye yavleniya i dispersnye sistemy v proizvodstve tekstil'nyh materialov i khimicheskih volokon.M.: «Sov’yazh Bevo» 2004.
- Chapurina M.A., Sintez i ispol'zovanie dlya modificirovaniya sinteticheskih volokon novyh ftorsoderzhashchih polimerov. Avtoreferat dissert. na soisk. uch. st. kand. khim.nauk. M.: MGTU im. A.N.Kosygina, 2007, 16 s.
- V.A. Volkov ,O.L. Bukanova, A.N. Zhironkin i dr., Primenenie modificirovannyh lateksov ftorsoderzhashchih polimerov dlya zashchitnoj poverhnostnoj propitki tkanej Trudy mezhdunarod. konf."Rubber-94" T.4, s.579-585. M:. 1994.
- V.A. Volkov, A.N. Zhironkin, L.A. Sorokina, Vliyanie kolloidno-himicheskih svojstv lateksov ftorsoderzhashchih polimerov i ftoruglerodnyh PAV na modifikaciyu tkanej.v. 1, Izv. Vuzov, ser. Tekhnologiya tekstil'n. prom. 1996, N1,s. 51-55
- V.A.Volkov, A.N.Zhironkin, L.A.Sorokina, Vliyanie kolloidno-himicheskih svojstv lateksov ftorsoderzhashchih polimerov i ftoruglerodnyh PAV na modifikaciyu tkanej.v. 2, Izv. Vuzov, ser. Tekhnologiya tekstil'noj promyshlennosti. 1996, N2,s.63-68
- A.F.Eleev, K.N.Bil'dinov, L.E.Deev, E.S.Klepikov, G.A.Sokol'skij, I.L.Knunyanc, Sposob polucheniya 2-sul'foftoridperftoralkanonov-3,Patent SU 683198 (1979).
- M.Yu.Pletnev, A.F.Eleev, A.F.Ermolov, Novyj tip polyarnoj gruppy, realizuyushchejsya u ftoruglerodnyh PAV, Kolloidnyj zhurnal, t. 52, s. 804-805 (1990).
- M.Yu.Pletnev, Khimicheskaya promyshlennost', N 1, s. 48-61(2000).
- M.Y.Pletnev in Surfactants: Chemistry, Interfacial Properties, Applications (Stud. Interface Sci., 13)/ Ed. By V.B.Fainerman, D.Möbius and R.Miller. – Amsterdam: Elsevier, 2001, pp. 1-97.
- A.F.Ermolov, A.F.Eleev, A.F.Benda, G.A.Sokol'skij, Acilirovanie «dvuhosnovnyh» SN-kislot, Zhurnal organicheskoj khimii, t. 23, N 1, s. 105-112 (1987).
- D.L.Katkov, N.K.Kutakova, A.F.Ermolov, A.F.Eleev, Sposob polucheniya metilpoliftoralkilketonov, Patent SU 1545510 (1989).
- A.F.Eleev, A.F.Ermolov, M.A.Kurykin i dr., Ftorsoderzhashchieβ-ketosul'fokisloty ili ih soli v kachestve PAV dlya processov, protekayushchih v vodnyh sredah, i sposob ih polucheniya, Patent RU 2005718 CI (1992).
- A.F.Eleev, I.Yu.Ermakova, Yu.P.Zdorov, A.V.Karasev, V.S.Polyakov, S.A.Frolova, S.S.Hohlov, Protivoiznosnaya prisadka k smazochnym sredam i toplivu, Patent RU 2206605 (2002).
- V.A.Volkov Micelloobrazovanie i adsorbciya difil'nyh veshchestv v nepolyarnyh sredah. Uspekhi kolloidnojkhimii, L.: 1991, s. 185-199
Recommended for publication by Prof. Alexander F. Eleev
Fluorine Notes, 2012, 83, 5-6