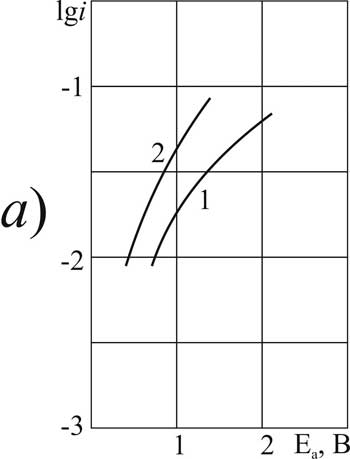Received: February, 2012
Fluorine Notes, 2012, 83, 3-4
Anodic behavior of substituted perfluorocarboxylic acids
O.N. Chechina *, V.V. Berenblit **, S.V. Sokolov ***, A.P. Tomilov
* Samara State Technical University,
** Federal State Unitary Enterprise NIISK after S.V.Lebedev,
*** CJSC "Anles"
e-mail: chechinao@yandex.ru
Abstract: Current-voltage studies of the anodic process on platinum and glassy carbon in solution of α-monochloroacetic acid as well as 2,3-dichloro- perfluoropropane acid, 3.4-dichloro-perfluorobutanoic and dichloro- perfluorobutanoic acids have been represented. The effect of pyridine additive and the process temperature have been shown. The work includes some proposals about the nature of the influence of pyridine additives in the Kolbe synthesis for halogen substituted perfluorocarboxylic acids and the possibility of using platinum and glassy carbon anodes.
Keywords: halogen substituted perfluorocarboxylic acids, pyridine, acetonitrile solutions, anodi polarization, the platinum anode, glassy carbon the anode, the mechanism of Kolbe synthesis, the free radicals, adsorbed radicals.
В Current-voltage studies for halogen substituted perfluorocarboxylic acids in the Kolbe synthesis have been of great interest. It is known that according to the theory of the Kolbe synthesis acids containing a single halogen substituent belong to the "abnormal" group. However, chlorine and fluorine, as substituents, are significantly different: the experiment showed that monochloroacetic acid is characterized by a higher anodic oxidation potential than the trifluoroacetic acid (TFA) [1].
Considering the smaller electronegativity of chlorine compared to fluorine (in the trifluoromethyl group of TFA) we can assume that the difficulties in this case are spatial in nature. The positive effect of pyridine additives including the implementation of the Kolbe cross-synthesis an acid mixture of "normal" and "abnormal" is experimentally established in preparative Kolbe synthesis for perfluorocarboxylic acids with the characteristics of the anomaly [2.3, Table 1]. It seems that rather high probability of cathodic elimination of a single halogen substituent (chlorine, bromine) on the cathode may be also important in the electrolysis for halogen substituted perfluorinated acids.
In this paper we investigated the nature of the effect of such substituents as chlorine, bromine and fluorosulfonic group on the anodic process in solutions of perfluorocarboxylic acids. The following acids were used as prototypes:
- monochloroacetic CF2ClCOOH (MCA), see Fig. 1 and 6;
- 2,3- dichloroperfluoropropanoic CF2ClCFClCOOH (DCP), see Fig. 2 and 7;
- 3,4- dichloroperfluorobutanoic CF2ClCFClCF2COOH (DCB), see Fig. 3;
- α-bromoperflouropropanoic CF3CFBrCOOH (BP), see Fig. 4 and 8;
- В fluorosulfonic acid FSO2CF2COOH (FS), Fig. 5.
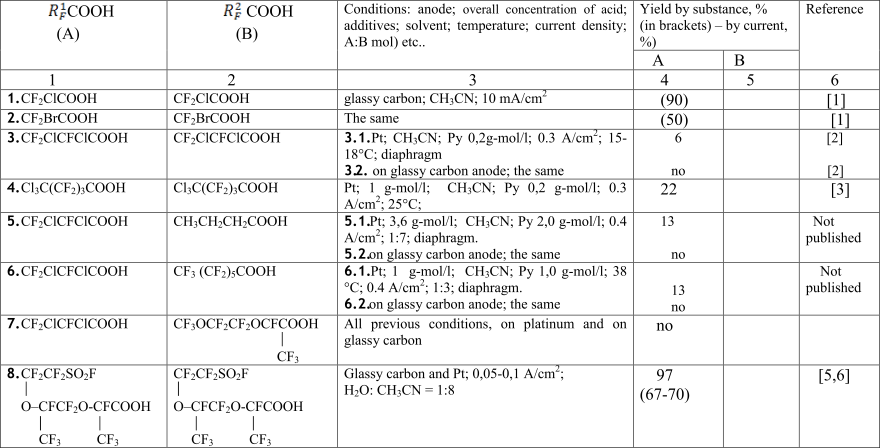
Table 1. Optimal conditions for the Kolbe dimers R1FВ - R2F synthesis involving perfluorinated halogen substituted and sulphofluoride-carboxylic acids.
The studies were conducted on platinum and glassy-carbon (GC) anode using acetonitrile solutions. The effect of temperature and additives of pyridine were considered. The method described previously [4] as for acids of tetrafluoroethylene was used for polarization measurements.
The results obtained allowed to suggest the ideas about the nature of adsorption processes in the Kolbe synthesis and the role of the cathode in this reaction.
Anodic polarization
It has already been established on the example of DCP acid that the introduction of chlorine into the molecule of perfluorinated acid does not cause qualitative changes in the character of polarization curves [5].
Research data of the effect of pyridine additives on current-voltage characteristics of platinum and GC at 20 В°C are shown in Figures 1-5, and temperature effect - in Figures 5-8.
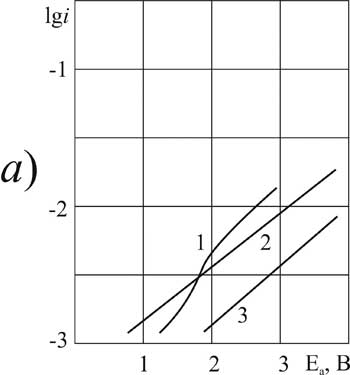
It is established that for investigated in this work О±- chlorosubstituted perfluorocarboxylic acids with one or two substituents at 20 В°C the introduction of a small amount (0.09 g-mol/L) of pyridine in solvent composition causes a significant anodic depolarization: on the platinum (about 2V), on the GC -anode (1 B). The form of the polarization curve of the Kolbe reaction remains classical as long as the concentration of pyridine reaches 3.2 mole / liter. On the platinum anode for DCB acid this trend holds even when concentration increases (Fig. 3a).
For other acids of this group the introduction of the additive (3.2 g-mol/L) causes the change in nature of the anodic process and its significant acceleration. At the same time the Tafel plots on the polarization curve disappear. For the lowest of the investigated chlorosubstituted acids (MCA) on platinum, and for the most high molecular weight (DCB) acid - on GC the anode potentials in solutions containing pyridine (3.2 g-mol/L) did not reach the value of 2.1 V required for the anodic condensation reaction (Fig. 1a, curve 3 and Fig. 3b, curve 7).
A further increase of the concentration of pyridine (6.3 g-mol/L ) on В platinum for all chlorosubstituted acids, and for DCB - on GC with the most smooth surface causes the strong inhibition of the oxidation process. It is caused by polymerization of pyridine on the surface of the anode. The form of the polarization curve under these conditions varies the same way for solutions of all acids of this group on GC. But very high concentrations in solutions of lower acids of MCA and DCP show a significant depolarization instead of inhibition. These acids are still actively involved in the anodic oxidation, the anode potential exceeds the value of 2.1 V (Fig. 1b and 2b, curves 8 and 5, respectively).
The analysis of polarization curves shows that the ratio of current density and the potential, where characteristic bend is observed and it is possible to carry out the Kolbe synthesis, depends on the nature of the anode and the nature of the chlorine substituted acid.  Thus, for the MCA acid "critical potential" containing pyridine (0.9 g-mol/L) is 3.1 V on platinum and 2 V on the GC -anode. Thus, the reaction on platinum proceeds under more severe conditions. For the more high molecular weight acids (DCP and DCB) in the same solvent the “critical potential" of the anode does not depend on the material it is made of.
Let's now separately consider the electrochemical properties of brominated acid BP. The choice of synthesis conditions for BP is more limited than for chlorine substituted acids. The process on GC-anode is more stable. On platinum the curves have the classical shape only in the presence of small amounts of pyridine - 0.1 mole/liter (Fig. 4). A further increase in additive concentration causes inhibition of oxidation of carboxylic BP acid: the angle of inclination "b" is increasing, but the position of the curve according to the scale of potentials gradually becomes the same as in the absence of additives.
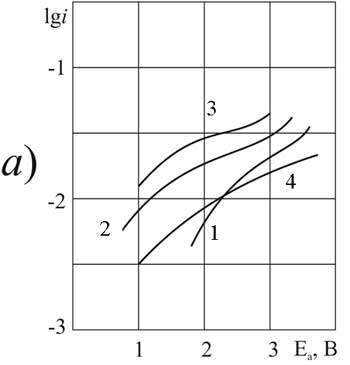
In contrast to halogen substituted perfluorocarboxylic acids the effect of pyridine on the oxidation of fluorosulfonic acid FS does not depend on the anode material (Fig. 5a and Fig. 5b). The characteristic form of the polarization curves indicates that the reaction can occur only in the absence or with a small pyridine additive(0.1 g-mol / l). When the concentration increases a significant depolarization of the anode potentials to less than 2.1 V appears. Poisoning of the anode surface here is not observed. Therefore, gum formation does not occur, although the Kolbe anodic dimerization is excluded.В
When analyzing the results we should take into account the fact that "normal" shape of polarization curves (for the Kolbe synthesis) does not always mean a successful reaction [7]. By the results obtained during the preparative synthesis we can also conclude that the presence of pyridine additives is an important condition for the anodic dimerization of halogen substituted fluorocarboxylic acids.
We conclude by considering the temperature effect for some ideas about the mechanism of pyridine influence on the reaction.
Table 2 shows the activation energy values of the anodic process for different acids and different anode materials. The values were calculated by temperature-kinetic method.
Table 2. The activation energy for the process of electrooxidation in acetonitrile solutions of substituted perfluorocarboxylic acids in the presence of pyridine. Concentration, g-mol/L: acid - 1; pyridine - 0.09.
|
Acid |
Anode | Activation energy, kJ / mol |
| CF2ClCOOH | Pt GC |
- 26,1 |
| CF2ClCFClCOOH | Pt GC |
16,7 - |
| CF2ClCFClCF2COOH | Pt GC |
46,0 17,7 |
| CF3CFBrCOOH | Pt GC |
18,8 13,6 |
| FSO2CF2COOH | Pt GC |
21,0 28,0 |
Analysis of polarization curves shows that the reaction of electrooxidation of halogen substituted fluorocarboxylic acids in the presence of pyridine on platinum occurs at lower anodic potentials than on GC. But the data presented in Table 2 for acids DCB and BP evidenced that the activation energy of the reaction on platinum is higher. This can be explained by the peculiarities of the parallel processes which affect the main reaction.
In В this case these processes are the adsorption and oxidation of pyridine on the surface active sites. Anodic activity of pyridine causes an increase of the activation energy of the main reaction as interaction of inert chlorine and bromine atoms on the anode in the composition of acid residue of perfluorocarboxylic acids with the platinum surface is hindered. This peculiarity of the reaction conditions for more high molecular weight and well adsorbed acid DCB on platinum is clearly visible.
The interaction of the halogen substituents with the surface of the platinum anode makes the reaction conditions more severe. At the same time the pyridine additive causes a softening effect.
Bromine substituted acid BP in the absence of pyridine on platinum does not enter into a reaction, appropriate favorable conditions can be created only in a narrow range of pyridine concentrations. However, this process should be successful in a wide range of pyridine concentrations on GC -anode which is less adsorbed.
Electrochemical characteristics of fluorosulfonic FS-acid is similar to chlorine substituted acids. But significant effect of pyridine on the activation energy of the process is not observed: the substituent does not interact with the anode surface. The results of polarization measurements suggest that synthesis in this case В successfully occurs on any of the anodes.
These results obtained suggest that the efficiency of the Kolbe synthesis depends on the characteristics of the anode microrelief. Orientation adsorption and molecule oxidation with dimerization of adjacent radicals occur on flat, smooth areas of the anode surface, which ranks second on energy characteristics [8]. The most active surface areas (ledges, corners and ribs of metal crystals) chemosorb halogens and halogen-containing fragments of the molecule (except fluorine). In this case, the other (flat) surface areas are screened by big enough fluorinated fragments repressed to the phase boundary.
This is an obstacle for the successful process. If the active areas are occupied by molecules of pyridine, the flat ones are released and carboxyl groups are oriented to the anode. On those areas of the anode, which ranks third on energy characteristics (depressions, basins), small-sized solvent molecules (H2O, CH3OH) can always be discharged. This ensures the current flow in the presence of the adsorbed molecules and acid radicals on the surface, but their energy is insufficient, and they can not overcome the energy barrier for dimerization.
Results
The Kolbe anodic dimerization involving halogen substituted perfluorocarboxylic acids is characterized by a complex combination of different adsorption processes - chemisorption, physical adsorption and orientation adsorption.
Optimum conditions for the synthesis should contribute to the orientation adsorption of the dipoles formed by the carboxyl groups of the molecules of fluorocarboxylic acids on flat В areas of the anode surface.
On the basis of the observed features the following conditions for preparative anodic condensation involving halogen perfluorocarboxylic acids are advised: an aprotic solvent (stabilizing carboxylic anions), the pyridine additive to the solvent (also improving dissociation and adsorption conditions) and low temperature. Using the diaphragm prevents elimination of the halogen substituents on the cathode.
Considering the steric hindrance for the formation of molecular dimers in solution and during electron transfer reactions, we can suggest that in this case (unlike with unsubstituted perfluorocarboxylic acids) the Kolbe synthesis preferably occurs under following conditions:
- on the platinum - by the Conway mechanism involving adsorbed radicals;
- on the glassy
carbon anode - the process involving free radicals is more likely to occur.
REFERENCES
2. Sokolov S.V., Berenblit V.V., Chechina O.N i dr. // Novosti ehlektrohim. organ. soedinenij 1986.ΙΧ Vsesoyuzn. Soveshch. po ehlektrokhim. organ. soedin.: Tez. dokladov. – M.: L'vov, 1986. – s. 191.
3. Chechina O.N. Issledovanie reakcii anodnoj kondensacii po Kol'be dlya poli- i perftorirovannyh kislot: Avtoref. dis. kand. khim. nauk – Sverdlovsk, 1969. – 21 s.
4. Chechina O.N., Berenblit V.V., Levin A.I. Vol'tampernye issledovaniya ehlektrosinteza Kol'be dlya triftoruksusnoj i perftormetoksioligooksaehtilenuksusnyh kislot // Zhurn. prikl. khimii, 1999. – T. 22. Vyp. 3. – s. 410-414.
5. Cherstkov V.F., Grinberg V.A., Sterlin S.R. // Izv. AN SSSR. Ser. Khim. – 1990. - №10. – s. 2448-2449.
6. WO 3731914 DE // S.A. 1989. – V.ΙΙΙ: 134912
7. Eskibaeva Ch.Z., Chechina O.N., Zapevalov A.Ya. i dr. // Zhurn. prikl. khimii. – 1990. – T. 63, - №4 – s. 845.
8. Antropov L.I. Teoreticheskaya ehlektrokhimiya. – M.: Vysshaya shkola, 1984. – 519 s.
Figures
 |
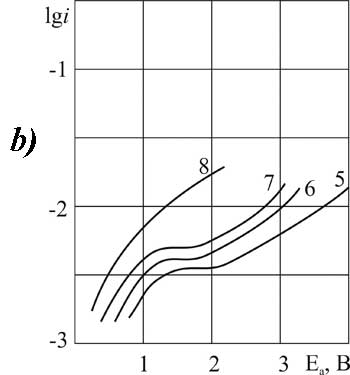 |
Figure 1. The polarization of platinum (a) and glassy carbon (b) anode at 20 В°C in 1 M solution of CF2ClCOOH in MeCN with pyridine additives, M: 1.5 - 0 2.6 - 0.09, 3.7 - 3.2, 4 8 - 6.3.
 |
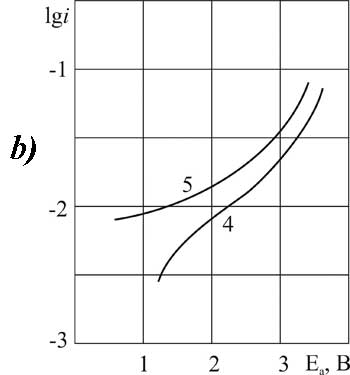 |
Figure 2. The polarization of platinum (a) and glassy carbon (b) anode at 20 В°C in 1 M solution of C2F3Cl2COOH in MeCN with pyridine additives, M: 1 - 0 2.4 - 3.2, 3.5, -6.3.
 |
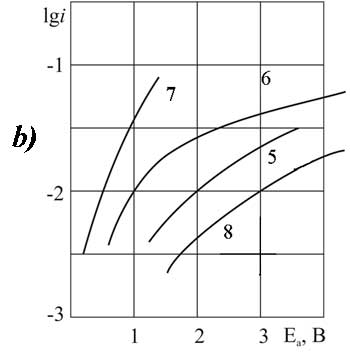 |
Figure 3. The polarization of platinum (a) and glassy carbon (b) anode at 20 В°C in 1 M solution of CF2ClCFClCF2COOH in MeCN with pyridine additives, M: 1,5 - 0; 2,6 - 0,1; 3,7 -3,2; 4,8 - 6,3.
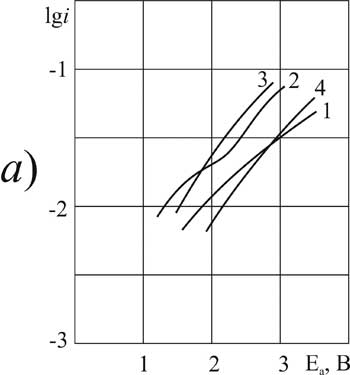 > > |
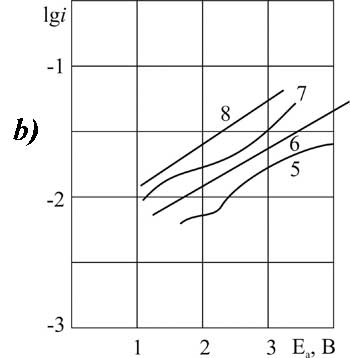 |
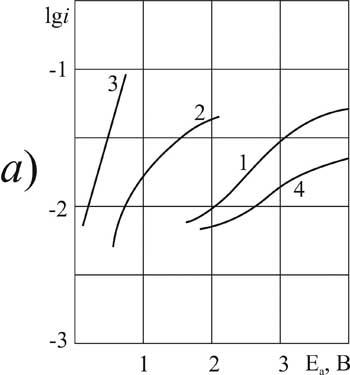
Figure 4. The polarization of platinum (a) and glassy carbon (b) anode at 20 В°C in 1 M solution of CF3CFBrCOOH in MeCN with pyridine additives, M: 1,5 - 0; 2,6 - 0,1; 3,7 -3,2; 4,8 - 6,3.
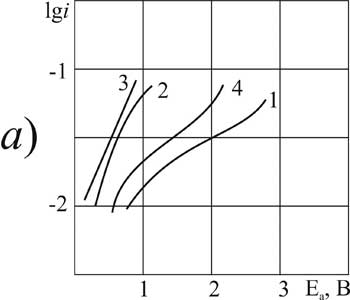 |
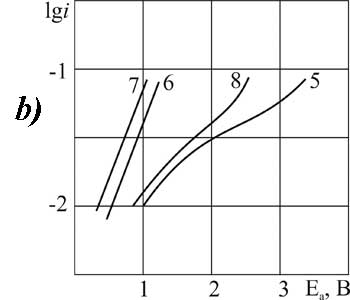 |
Figure 5. The polarization of platinum (a) and glassy carbon (b) anode at 20 В°C in 1 M solution of FSO2CF2COOH in MeCN with pyridine additives, M: 1,4,5,8 - 0,1; 2,6 - 3,2; 3,7 - 6,3.
|
|
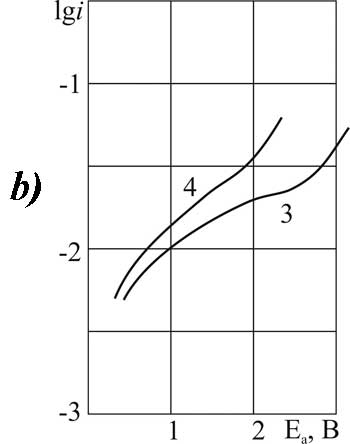 |
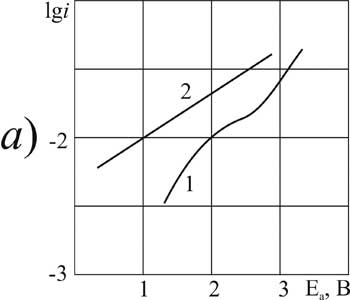
Figure 6. The polarization of platinum (a) and glassy carbon (b) anode in MeCN in 1M solution of CF2ClCOOH + 0,1 Рњ of pyridine at t В°C: 1,3 - 20, 2.4 - 40.
|
|
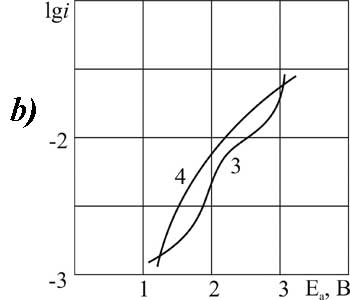 |
Figure 7. The polarization of platinum (a) and glassy carbon (b) anode in MeCN in 1M solution of C2F3Cl2COOH + 0,1 Рњ (3,4)or 3.2 M (1.2) of pyridine at t В°C: 1,3 - 20, 2, 4 - 40.
 |
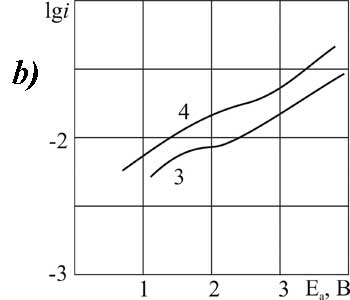 |
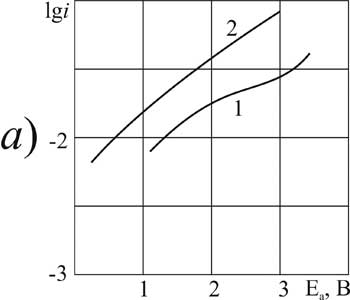
Figure 8. The polarization of platinum (a) and glassy carbon (b) anode in MeCN in 1M solution of CF3CFBrCOOH + 0,1 Рњ of pyridine at t В°C: 1,3 - 20, 2, 4 - 40.
Recommended for publication by prof. A. Zapevalov
Fluorine Notes, 2012, 83, 3-4