Received: May, 2012
Fluorine Notes, 2012, 83, 1-2
Persulfate oxidation of mono- and dichlorinated benzenes and biphenyls in the presence of C3F7• radicals
T.I. Gorbunova, M.G. Pervova, A.Ya. Zapevalov, V.I. Saloutin
I.Ya. Postovsky Institute of Organic Synthesis, Russian Academy of Sciences, 22/20 S. Kovalevskoy/Akademicheskaya
St., 20990 Ekaterinburg, Russian Federation
Tel.: +7343362 34 87; fax: +7343374 59 54.
e-mail:
gorbunova@ios.uran.ru
Abstract: Chloroaromatic compounds are known as persistent organic pollutants (POPs). For remediation of contaminated soils and groundwater from these POPs inorganic persulfates are used as reagents of the in situ chemical oxidation (ISCO). The same persulfates are applied for oxidative decarboxylation of carbon acids, their salts and esters. In this study, we researched the reactive abilities of mono- and dichlorinated benzenes (chlorobenzene (CBz), o- and p-dichlorobenzenes (o-DCBz and p-DCBz)) and biphenyls (monochlorobiphenyls (PCB-Cl) and dichlorobiphenyls (PCB-Cl2)) with potassium persulfate in the presence of potassium salt of heptafluorobutanoic acid. The studied processes are not selective, different groups of isomeric products are formed. Their structures were identified by means of gas chromatography with a mass-spectrometer detector (GC-MC). The major products of reactions are compounds with heptafluoropropyl groups. The most reactive compounds are monochlorinated in comparison with the dichlorinated substrates. The developed techniques can be used as an alternative method of chemical transformation of the POPs for their further degradation.
Keywords: in situ chemical oxidation, mono- and dichlorinated benzenes and biphenyls, persulfate anion, sulfate radical anion, heptafluoropropyl free radical
1. Introduction
Inorganic persulfates are becoming increasingly popular reagents that are studied in order to be used for remediation of contaminated soils and groundwater [1]. Persulfate anion effect is based on rather simple activation as a result of which active particles can be formed in pre-surface layers of contaminated matrixes. The particles are capable of destroying persistent organic pollutants (POPs). The inorganic persulfates are regarded as reagents for the in situ chemical oxidation (ISCO).
Several ways of radical activation from persulfates in aqueous medium at different pH are known [2-9]. In aqueous alkaline medium the persulfate anion (S2O82-) is converted to sulfate radical anions which can proceed through propagation reactions to give rise to the same reactive species. These processes are carried out at ambient and higher temperatures and are summarized in Eqs. 1-7 [7,10,11]:
S2O82- → 2SO4•-(1)
SO4•- + HO - → HSO4 - + HO • (2)
HO • + HO • → H2O + ½ O2 (3)
S2O82- + HO • → HSO4 - + SO4•- + ½ O2(4)
S2O82- + HO • → S2O8•- + HO - (5)
SO4•- + HO • → SO42- + ½ O2 (6)
SO4•- + S2O82- → SO42- + S2O8•- (7)
The formation of the in situ reactive species leads to competing processes in reactive zones. The sulfate radical anion (SO4•-) possessing strong oxidizing properties interacts with organic molecules by three different mechanisms: an electron transfer from SO4•- to the organic substrate, hydrogen abstraction from the organic structure, addition and elimination reactions with alkenes and aromatic compounds.
In general, published results are related to studies of the influence of persulfates oxidizing properties on only one organic substrate. The works devoted to synergism of two and more organic compounds being in a reactive zone with the persulfate are practically inexistent. However, the presence of a second compound and the influence of S2O82- on it can lead to a change of the reactive ability of all the substrates.
It is known that inorganic persulfates are initiators of oxidative decarboxylations of organic acids, their salts and esters [12-14]. These reactions are typical of halogen containing compounds. For example, salts of aliphatic perfluorocarbon acids were decarboxylated for 50 min in an aqueous medium at 95 oC under the influence of K2S2O8. The result of the reaction is dimeric perfluoroalkanes [15]:
2RFCOOK → 2RFCOO• → 2CO2 + 2RF • → RF - RF(RF = F-Alkyl)(8)
It may be suggested that the formation of a free radical RFCOO• is connected with a transfer of a non-coupled electron from the formed in situ particles SO4•- to a carboxyl group. The subsequent ejection of carbon dioxide gas creates favorable conditions for the formation of highly active radicals RF• participating in the recombination.
The purpose of our research is to model oxidative processes of chloroaromatic compounds under the influence of an inorganic persulfate in the presence of fluorine-containing reagents. Mono- and dichlorinated benzenes and biphenyls belonging to POPs are used as oxidation objects. Potassium salt of heptafluorobutanoic acid (C3F7COOK) is used as fluorine-containing reagent. Among persulfates, K2S2O8 is chosen. It possesses rather low solubility in water and higher half-life temperature in comparison with those of Na2S2O8 [14,15]. The obtained results can serve as a possible method of turning the toxic chloroaromatic compounds into nontoxic (with a little toxicity) or a method of preparing them for further degradation.
2. Materials and methods
2.1. Chemicals
Benzene (cp grade, Russia), chlorobenzene (99%, Alfa Aesar), o- and p-dichlorobenzenes (99% and 99+% accordingly, Alfa Aesar), aniline (cp grade, Russia), 3- and 4-anilines (cp grade, Russia), heptafluorobutanoic acid (cp grade, Russia), potassium hydroxide (cp grade, Russia), potassium persulfate (cp grade, Russia) were used. Mono- and dichlorobiphenyls were synthesized in accordance with the Gomberg-Bachmann-Hey reaction as described in the article [16], i-amylnitrite was synthesized according to a known procedure [17].
2.2. Procedures
Potassiumsaltofheptafluorobutanoicacid. In all cases the potassium salt of heptafluorobutanoic acid (C3F7COOK) was obtained via mixing equimolar quantities of potassium hydroxide (KOH, 40 wt % in water) and heptafluorobutanoic acid. Then KOH was added to the active mass in order for pH to be nearly 10-11. In the performed reactions the molar excess of C3F7COOK in relation to the chloroorganic object corresponds to the amount of carbon atoms not substituted by chlorine atoms in the aromatic structure.
A typical procedure of interaction of chloroaromatic compounds with the C3F7COOK in the presence of K2S2O8. To the aqueous solution of 0.25 mol prepared in situ C3F7COOK 5.6 g (0.05 mol) of chlorobenzene was added under agitation. The mixture was heated up to 50 °C and 10.0 g (0.037 mol) of K2S2O8 was added. Then the active mass was heated up to 95 oC and cooled down to 40-50 °C. The procedure of adding K2S2O was repeated until 40.5 g (0.15 mol) of potassium persulfate was introduced. Then the active mass was agitated during 2 h at 95 oC and cooled down to 40-50 °C. Then 100 ml of water was added to dissolve inorganic salts. If necessary, the amount of water can be increased. After that the reaction mass was cooled to room temperature and extracted by chloroform (3 x 30 ml).
2.3. Analysis
Measurements on gas chromatography with mass-spectrometer detector (GC-MS) were made using Agilent 7890A MS 5975C Inert XL and quartz capillary column HP-5-MS (30 m x 0.26 mm x 0.25 μm) with film of stationary phase 0.25 mm thick (polymethylsiloxane, 5% of grafted phenyl groups). Electronic ionization 70 eV, scan rate 1 s-1 of the full ion current in the mass range 20-1000 amu. The gas carrier was helium, split ratio was 1:50. The initial temperature of the column was 40 °C (isotherm 3 min), the heating was performed with a speed of 10 °C min-1 to 290 °C (isotherm 40 min). The temperature of the evaporator was 250 °C, of the heater sleeve - 280 °C, of the source - 230 °C, of the quadrupole - 250 °C.
The quantitative estimation was carried out by internal normalization. In this work compound structures are presented whose content in the resulting mixtures of products was higher than 0.1%.
3. Results and discussion
3.1. Chlorobenzene (CBz)
The CBz underwent complete conversion interacting with five-fold excess of C3F7COOK in the presence of K2S2O8 with the formation of a multicomponent mixture of reactionary products. Chemical structures of the resulting compounds were established by scanning every chromatographic peak in the GC-MS conditions and subsequent reconstruction of fragmentary ions in mass spectra (Scheme 1). Their relative contents are presented in the Table.
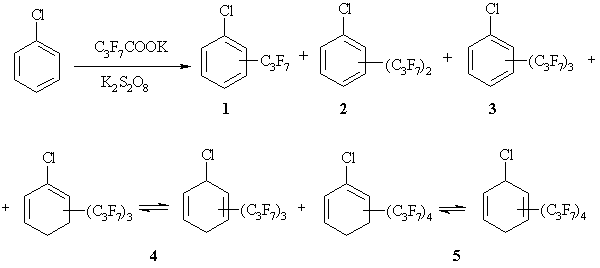
Scheme 1. The mixture of the compounds obtained from interaction of CBz with C3F7COOK in the presence of K2S2O8.
It should be noted that the chemical interaction of CBz with C3F7COOK in the presence of K2S2O8 is not a selective process and the formation of groups of various isomers is possible. For example, the chloro(heptafluoropropyl)benzene 1 in allocated mixture is presented by three isomers (o-, m-, p-) which were obtained via hydrogen abstraction in the form of H• under the attacking effect of a free radical C3F7• formed in situ to carbon atom in an aromatic ring. However, this formal reactionary pathway was not the main process.
The chloro[di(heptafluoropropyl)]benzenes 2 made the major contribution to the interaction products (four isomers). In general, they are results of two hydrogen abstractions in the form of H• under the attacking effect of two free radicals C3F7•. Unfortunately, the arrangement of heptafluoropropyl groups in a benzene ring is not known, GC-MS does not help to establish their positions. However, it is known that the primary centers of radical attacks on monosubstituted benzenes containing an electron-withdrawing substituent are o- and p-positions [5,18]. The chloro-2,4-(diheptafluoropropyl)benzene 2 might be the main compound in the total mass of the reactionary products.
The chloro[tris(heptafluoropropyl)]benzenes 3 made up a rather small amount in the resulting mixture, the mechanism of their formation is similar to the reaction for compounds 1 and 2.
Conclusion about non-aromatic nature of compounds 4 and 5 is based on tentative GC-MS. The molecular ions of the compounds 4 and 5 were two units higher than in the aromatic substrates. There are differences in fragmentation of heptafluoropropyl substituents. In the case of aromaticity of the cyclic fragment in a molecule the cleavage of C3F7 groups proceeded as the formation of [C2F5]+ ions with m/z 119 at first and the ion peak [M-C2F5]+ was basic in mass spectra for the compounds 1-3. In the case of the structures 4 and 5 the [C3F7]+ ions with m/z 169 occurred in mass spectra and the peak of [M-C3F7]+ ion was basic. The [M-C2F5]+ peak possessed low intensity (3-4%). These conclusions make it possible to assume the cyclohexadienyl structure of cyclic fragment in molecules.
Earlier the formation of cyclohexadienyl particles as intermediates was repeatedly reported in radical reactions of aromatic compounds [2,3,5,19,20]. In the presence of molecular oxygen they formed the peroxyl radicals C-OO•. However, the presence of an electron-withdrawing substituent in an aromatic ring, for example, the chlorine atom or trifluoromethyl group, makes this process impossible. For the formation of C-OO• the excess electronic density in the aromatic substrate is required [5].
Any of these stabilizing pathways does not explain the formation of the compounds 4 and 5. It might be necessary to pay attention to the steric factors accompanying the interaction of CBz with C3F7COOK in the presence of K2S2O8. In Scheme 2 the pathways of the formation of some products by C3F7• attacks on o-, m- and p-positions in CBz are presented. It is important to note that the interaction between C3F7• and CBz to m-position of the aromatic ring leads to a possible resonance structure A. In order to stabilize it, hydrogen abstraction in the form of H• is necessary. However, owing to the specific arrangement of the leaving group between the chlorine atom and the rather bulky heptafluoropropyl substituent [21], the hydrogen abstraction becomes impossible and the compound with the cyclohexadienyl structure B is formed as a result of disproportion.
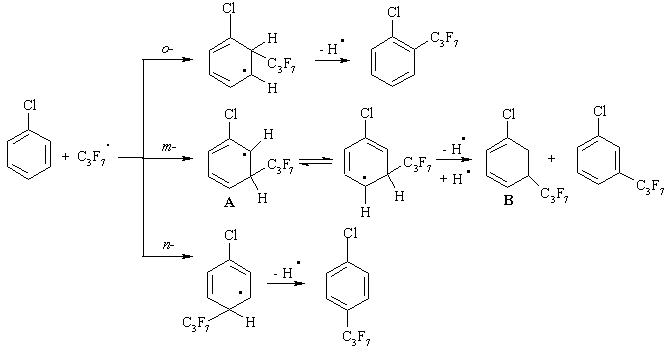
Scheme 2. Possible pathways of interaction between CBz and free radicals C3F7•.
Following this assumption, only chloro[di(heptafluoropropyl)]cyclohexadienes can be formed further from the structure B via attacks on the 4- and 5-positions. However, this type of compounds in the resulting mixture was not identified. From all the presented structural formulas in Scheme 2, the key compounds for the formation of the product 4 can be 1-chloro-2-heptafluoropropyl- and 1-chloro-3-heptafluoropropylbenzene (Scheme 3 in an example 1-chloro-2-heptafluoropropylbenzene) and for the formation of the product 5 can only be 1-chloro-2-heptafluoropropylbenzene.
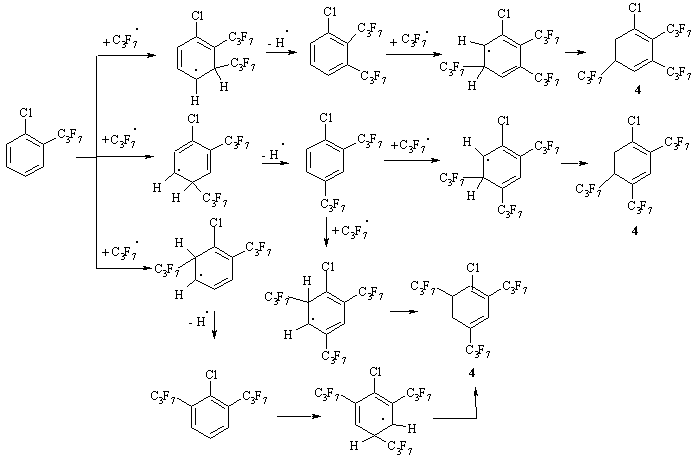
Scheme 3. Possible pathways of the formation of cyclohexadienyl derivaties based on CBz.
It should be noted that fragmentations of 1,3- and 1,4-cyclohexadienes in GC-MS conditions (70 eV) are similar and, therefore, it is necessary to consider the process of possible isomerization of double bonds from the 1,3- to 1,4-positions in the aromatic cycle. The isomerization of the double bonds is characteristic of analogues obtained by using other chloroaromatic substrates.
3.2. Dichlorobenzenes (o-DCBz and p-DCBz)
Dichlorobenzenes (o- and p-) under the conditions of the studied processes possess high reactivity but they do not undergo complete conversion. The o-DCBz and p-DCBz were found in the resulting mixture, their contents were 1.5 and 7.3 %, respectively. Earlier by Density Functional Theory (DFT) calculations it was found that among dichlorobenzenes the p-DCB is more stable in comparison with its o- and m-isomers [22] as verified by our results.
As well as in the case of the CBz a multicomponent mixture of reactionary products is a result of interaction of the o-DCBz and p-DCBz with fourfold excess of C3F7COOK in the presence of K2S2O8 (Scheme 4, Table). The compounds 6 and 7 were detected as the major DCBz's derivatives similarly to the reactive pathways for the CBz. For the o-DCBz the compounds 6 and 7 had two possible isomers. In the case of the p-DCBz the only possible compound 6 was registered - 1,4-dichloro-2-heptafluorobenzene and two isomers of the compound 7 were detected.
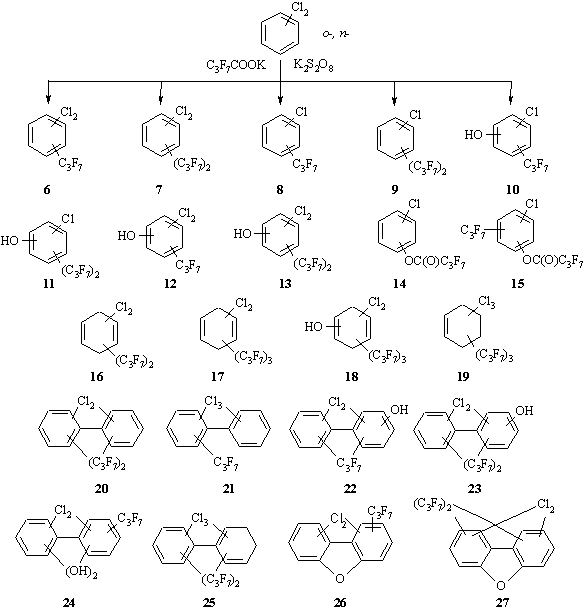
Scheme 4. The mixtures of the compounds obtained from interaction of o- and n-DCBzs with C3F7COOK in the presence of K2S2O8.
The compounds 8 (for o- and p-DCBzs) and 9 (only for o-DCBz) correspond to the 1 and 2 structures formed from CBz. However, the assumption about homolysis of the CAr-Cl bond under the effect of radical C3F7• with further chlorine abstraction in the form of Cl• and with the formation of a new CAr-Cl bond is not right. The homolytic cleavage of CAr-Cl bond in chlorobenzenes is a highly power-input process [23] and reactive conditions chosen by us do not promote its realization [24,25. It is reasonable to assume that the formation of the monochlorinated compounds 8-11,14,15 was a result of several competing processes in the reactive mass: the addition and elimination of radicals, disproportion, isomerization, etc.
It was shown before that under photolysis initiation of SO4•- the fluorine-containing phenols are formed from trifluoromethylbenzene [5]. On the basis of the Hammett constants it is established that a preferable pathway of the formation of hydroxyl derivatives was the addition of a sulfate ion-radical to the aromatic substrate and its subsequent stabilization by means of the elimination of SO42- and H+ under the influence of water. In the absence of the photolysis initiation of SO4•- the most probable pathway of the formation of the hydroxyl derivatives 11-13 is the effect of HO• formed in situ (Eq. 2) and the stabilization of the aromatic ring under hydrogen abstraction H• as was shown in the article [19]. It should be noted that the compound 12 was formed for the p-DCBz only.
The derivatives 14 and 15 representing esters of phenol and heptafluorobutanoic acid are most likely to have been obtained as a result of the attack of C3F7COO• radicals formed in situ (Eq. 8) on one of carbon atoms in the aromatic cycle. An alternative two-stage way - the formation of phenols under the influence of HO• with hydrogen abstraction and further esterification by heptafluorobutanoic acid - is hardly possible. The generation of heptafluorobutanoic acid in situ is doubtful since pH in the beginning of the process is strong alkali and esterification catalysts are absent.
The presence of the compounds 16 in the reactive products formed from only o-DCBz does not contradict assumptions formulated for derivatives CBz. It is in good agreement with the fact that the compounds 16 based on p-DCBz are absent. The formation of the compounds 17-19 does not correspond with our assumptions and requires additional research.
It is known that for the stabilization of hydroxycyclodienyl and aryl radicals their dimerization is required with the formation of substituted biphenyls [5,20]. It is thus clear why the compounds 20-25 are present in the resulting mixture. It should be noted that all the derivatives 20-25 are a result of dimerization of mono- and dichlorobenzenes but among them there is no product preserving the quantity of chlorine atoms of the initial substrates. Also among the compounds 20-25 there are no products formed for both DCBzs simultaneously.
The dichlorodibenzofuranes (PCDF) 26,27 were detected in the resulting mixture for n-DCBz only. A possible mechanism of the formation of PCDF was proposed in the review [26], formation of the di(trifluoromethyl)dibenzofurane was noted in the article [5].
Thus with an increase of chlorine atoms in the aromatic ring the number of typical products of oxidation increases considerably upon going from CBz to DCBzs.
3.3. Monochlorobiphenyls (PCB-Cl)
PCB-Cl are components of some commercial mixtures of polychlorobiphenyls (PCB), for example, Clophen A 30 [27] or Delor 103 [28]. Their structure is similar to CBz and contains electron-donating phenyl substituent. In order to establish similarities and differences in the reactivity of CBz and PCB-Cl, a mixture of monochlorinated congeners of PCB (PCB-Cl) was synthesized via aryl-aryl coupling of 3-chloroaniline with benzene in the presence of i-amylnitrite (i-Amyl-NO2) formed according to [16] with dominating content of PCB 2 (3-chlorobiphenyl):
ClC6H4NH2 + C6H6 → C6H5-C6H4Cl (9)
For monochlorinated biphenyls an example of full mineralization is known of the 2-chlorobiphenyl (PCB 1) under the influence of SO4•- initiated by excess Fe2+ ions from peroxymonosulfate (KHSO5) [9]:
C12H9Cl + 28.5KHSO5 → 12CO2 + 4.5H2O + 28.5KHSO4 + Cl- (10)
Analysis of the mixture of products synthesized by interaction of PCB-Cl with ninefold excess of C3F7COOK in the presence K2S2O8 showed this congeners group undergoes complete conversion. Eight types of derivatives were obtained among which the major compounds were chloro(diheptafluoropropyl)benzenes 29 as in the case of CBz (Scheme 5, Table). It is important that among derivatives the compounds 31 and 32 are not chlorinated. Their toxicity might be below that of chloroaromatic substrates.
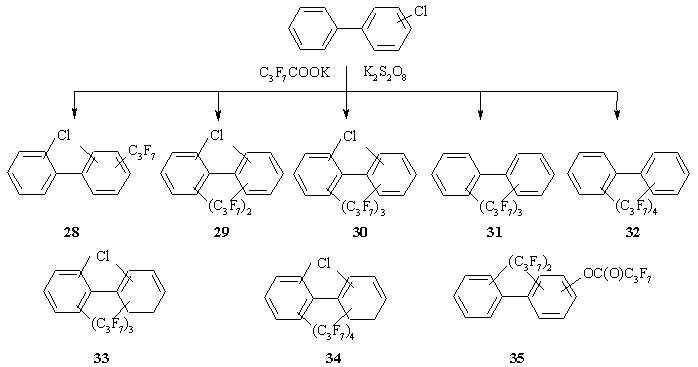
Scheme 5. The mixture of compounds obtained from interaction between PCB-Cl and C3F7COOK in the presence of K2S2O8.
From the performed analysis it may be noted that PCB-Cl are similar to CBz as regards their reactivities.
3.4. Dichlorobiphenyls (PCB-Cl2)
In order to establish the reactivity of dichlorinated congeners of PCB, two types of derivatives were synthesized: one with an arrangement of two chlorine atoms in one aromatic ring of biphenyl structure (from aniline and 1,2-dichlorobenzene):
C6H5NH2 + Cl2C6H4 → C6H5-C6H3Cl2(11)
and the other one with an arrangement of two chlorine atoms, one of them in each aromatic cycle:
ClC6H4NH2 + ClC6H5 → ClC6H4-C6H4Cl(12)
The structures of the compounds based on PCB-Cl2 after interaction with eightfold excess of C3F7COOK are presented in Scheme 6, their relative contents are shown in Table. In both series the incomplete conversion of PCB-Cl2 is established. The PCB-Cl2 were found in the resulting mixture, their contents were 6.4 and 9.3% according to Eqs. 11 and 12. A major contribution in the reactive products is made by dichloro(heptafluoropropyl)biphenyls 36 as in the case of o- and p-DCBz.
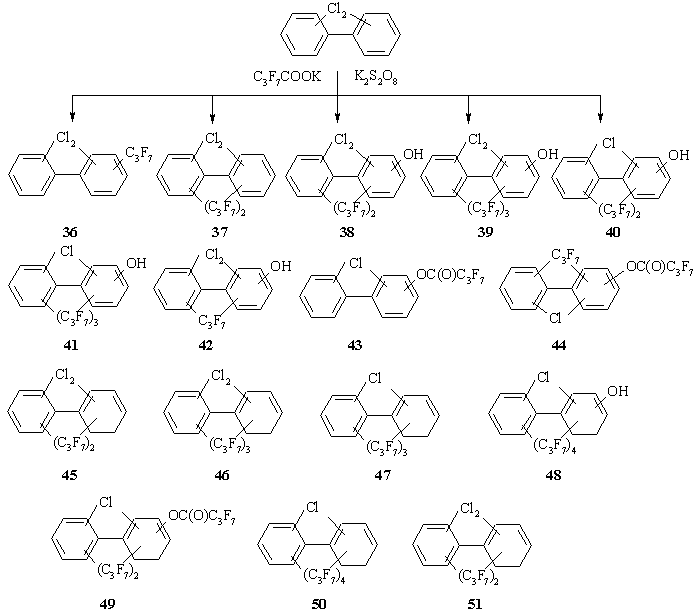
Scheme 6. The mixtures of compounds obtained from interaction between PCB-Cl2 and C3F7COOK in the presence of K2S2O8.
Summarizing the results obtained for this series, it may be noted that the reactivity of PCB-Cl2 is similar to that of DCBzs.
3.5. GC-MS
The mass spectra of all the identified compounds display peaks of molecular ions having different relative intensities (from 10 to 100%). Also peaks of the ions [M-F]+, [M-HF2]+ and peaks characteristic of the cleavage of the heptafluoropropyl group [C3F7]+, [C2F5]+, [CF3]+ were detected.
The basic peak displayed in the mass spectra of the derivatives 1-3,6-9,20,26-32,36,37,47 is that of the [M-C2F5]+ ion. Other ion peaks possess low relative intensity (up to 10%). With an increase in the number of heptafluoropropyl groups further cleavage of molecular ions occurs as consecutive losses of all heptafluoropropyl groups. After that the loss of chlorine atoms is observed.
The highly intensive or basic peak in the mass spectra of the derivatives 4,5,16-19,25,33,34,45,46,50,51 is that of the [M-C3F7]+ ion. The peak of [M-C2F5]+ possesses very low relative intensity or might be totally absent. The peaks of the [C3F7]+, [C2F5]+, [CF3]+ ions have high intensity and in a number of cases the peak of [C3F7]+ is basic.
In the mass spectra of the derivatives 14,15,35,43,44,49 the peaks of the [M-COC3F7]+ ion are detected and in some cases they are basic. In the mass spectra of the derivatives with C3F7 and C3F7C(O)O groups the loss of the C2F5 group occurs at first followed by the loss of the C3F7C(O) group.
In general, for the derivatives 10-13,21-24,38-42,48 the ion peaks [M-C2F5]+ are basic. For similar derivatives containing two and more heptafluoropropyl groups characteristic peaks are peaks [M-119-166]+ corresponding to the loss of [M-2C2F5-F-CO]+.
3.6. About practical application
Summarizing the results of the presented research, we conclude that the main process realized upon interaction of mono- and dichlorinated benzenes and biphenyls with C3F7COOK in the presence of K2S2O8 is radical exchange of hydrogen in the form of H• for heptafluoropropyl group in the form of C3F7•. In none of the experiments did the formation of perfluoroalkane C6F14 occur (see Eq. 8). It may seem that such complication of initial substrates by means of additional substituents will potentially lead to more difficult processes of subsequent degradation of new derivative's mixtures. However, it is a simplified view on problems of POP degradation.
Firstly, the influence of fluorine atoms and fluoroalkyl groups bonded with aromatic cycle as C-F and C-F-Alkyl, respectively, on toxic levels of organic compounds is still not clear [29,30]. Secondly, the developed procedures make it possible to obtain aromatic compounds with perfluoroalkyl groups which possess lower stability in comparison with substrates containing the CAr-F bond [31]. Arrangements of substituents in the aromatic ring and their influence on each other are very important as well. Thirdly, there are instances of a synergetic effect of chlorine- and fluorine-containing compounds on a decrease of their general toxicity. For example, perfluorooctane acid (PFOS), triclosan and 2,4,6-trichlorophenol are known. Individually or in binary mixtures they behave antagonistically towards growth inhibition of the green alga Pseudokirchneriella subcapitata [32]. But the ternary mixture of PFOS, triclosan and 2,4,6-trichlorophenol does not possess such an effect. Fourthly, there is mild hydrolysis of 2-trifluoromethylphenols to salicylic acid in water as self-remediation of water under the influence of microorganisms [33] and the C3F7 group is very similar to trifluoromethyl substituent. Finally, processes of effective mineralization of fluorocarbon acids in the presence of persulfates in hot water [34] and of perfluorooctanoate and perfluorooctane sulfonate in aqueous medium in the presence of humic acid photolysis [35] are known. In both cases there is defluorination in alkyl chain of initial substrates.
Thus the addition of perfluoroalkyl substituents to the aromatic cycle creates new possibilities for the degradation of chloroaromatic compounds using more various methods, both chemical and biological.
4. Conclusions
The presented study leads to the following conclusions:
- interaction of mono- and dichlorinated aromatic compounds with C3F7COOK in the presence of K2S2O8 is not a selective process;
- major products of reactions are compounds with heptafluoropropyl groups as a result of hydrogen abstraction under the influence of C3F7• free radicals formed in situ;
- the reactivity of mono- and dichlorinated benzenes decreases as CBz > o-DCBz > p-DCBz;
- the reactivity of mono- and dichlorinated biphenyls decreases as PCB-Cl > PCB-Cl2;
- the reactivity of CBz and PCB-Cl is similar. They undergo complete conversion;
- among PCB-Cl2 the structures with two chlorine atoms in one aromatic ring possess higher reactive ability in comparison with PCB-Cl2 having two chlorine atoms in different aromatic rings;
- - the developed procedures can be used to decrease the toxicity of low-chlorinated aromatic pollutants or to convert them for further destruction, for example, by microbiological methods.
Table. Results of interaction of mono- and dichlorinated aromatic compounds with C3F7COOK in the presence of K2S2O8
|
Compound no. |
Content in mixture, % |
Number of isomers |
Molecular ion m/z / rel. int., % |
MS m/z / rel. int., % [abundant ion] |
|
Compounds based on CBz |
||||
|
1 |
12.7 |
3 |
280/22 |
261/4 [M-F]+, 241/1 [M-HF2]+, 211/2 [M-CF3]+, 161/100 [M-C2F5]+, 126/7 [M-C2F5-Cl]+, 69/3 [CF3]+ |
|
2 |
42.0 |
4 |
448/13 |
429/7 [M-F]+, 409/2 [M-HF2]+, 379/1 [M-CF3]+, 329/100 [M-C2F5]+, 310/1 [M-C2F5-F]+, 210/52 [M-2C2F5]+, 160/5 [M-2C2F5-CF2]+, 125/6 [M-2C2F5-CF2-Cl]+, 169/3 [C3F7]+, 119/5 [C2F5]+, 69/6 [CF3]+ |
|
3 |
1.5 |
1 |
616/4 |
597/9 [M-F]+, 547/1 [M-CF3]+, 497/100 [M-C2F5]+, 378/7 [M-2C2F5]+, 343/2 [M-2C2F5-Cl]+, 205/4 [M-2C2F5-2CF3]+, 169/2 [C3F7]+, 119/1 [C2F5]+, 69/4 [CF3]+ |
|
4 |
9.1 |
3 |
618/19 |
599/12 [M-F]+, 579/3 [M-HF2]+, 499/3 [M-C2F5]+, 449/61 [M-C3F7]+, 329/100 [M-C3F7-HC2F5]+, 210/20 [M-C3F7-HC2F5-C2F5]+, 169/79 [C3F7]+, 119/8 [C2F5]+, 69/46 [CF3]+ |
|
5 |
14.8 |
1 |
786/2 |
767/5 [M-F]+, 667/4 [M-C2F5]+, 617/3 [M-C3F7]+, 497/3 [M-C3F7-HC2F5]+, 429/23 [M-C3F7-C2F5-CF3]+, 329/17 [M-C3F7-C2F5-C3F7], 169/100 [C3F7]+, 119/3 [C2F5]+, 69/23 [CF3]+ |
|
Total: 80.1 |
||||
|
Compounds based on o-DCBz/p-DCBz |
||||
|
6 |
30.5/32.2 |
2/1 |
314/35 |
295/2 [M-F]+, 275/2 [M-HF2]+, 245/1 [M-CF3]+, 195/100 [M-C2F5]+, 160/6 [M-C2F5-Cl]+, 125/12 [M-C2F5-2Cl]+, 119/2 [C2F5]+, 69/6 [CF3]+ |
|
7 |
7.2 /12.5 |
2/ 2 |
482/21 |
463/13 [M-F]+, 443/3 [M-HF2]+, 413/1 [M-CF3]+, 363/100 [M-C2F5]+, 344/12 [M-C2F5-F]+, 244/61 [M-2C2F5]+, 174/7 [M-2C2F5-2Cl]+, 169/9 [C3F7]+, 119/7 [C2F5]+, 69/11 [CF3]+ |
|
8 |
0.4/0.3 |
1/1 |
280/25 |
similar to fragmentation of compounds 1 |
|
9 |
5.8/- |
1/- |
448/15 |
similar to fragmentation of compounds 2 |
|
10 |
-/3.3 |
-/2 |
296/38 |
227/2 [M-HF2]+, 176/100 [M-C2F5]+, 147/6 [M-C2F5-HCO]+, 113/7 [M-C2F5-HCO-Cl]+, 119/2 [C2F5]+, 69/8 [CF3]+ |
|
11 |
1.1/4.5 |
1/3 |
464/22 |
445/5 [M-F]+, 425/1 [M-HF2]+, 345/100 [M-C2F5]+, 179/9 [M-2C2F5-F-CO]+, 169/1 [C3F7]+, 119/3 [C2F5]+, 69/8 [CF3]+ |
|
12 |
-/1.3 |
-/1 |
330/100 |
291/1 [M-HF2]+, 211/3 [M-C2F5]+, 160/59 [M-C3F7]+, 132/34 [M-C3F7-CO]+, 169/5 [C3F7]+, 119/8 [C2F5]+, 69/2 [CF3]+ |
|
13 |
0.2/0.7 |
1/1 |
498/52 |
429/8 [M-F]+, 379/69 [M-C2F5]+, 329/52 [M-C3F7]+, 300/4 [M-C3F7-HCO]+, 212/100 [M-2C2F5-F-HCO]+,181/29 [M-2C2F5-CF2-CO]+, 147/9 [M-2C2F5-CF2-CO-Cl]+, 169/26 [C3F7]+, 119/17 [C2F5]+, 69/6 [CF3]+ |
|
14 |
5.0/1.0 |
1/1 |
324/100 |
289/6 [M-Cl]+, 127/69 [M-COC3F7]+, 99/52 [M-COC3F7-CO]+, 73/15 [M-COC3F7-CO-C2H2-Cl]+, 169/29 [C3F7]+, 119/8 [C2F5]+, 69/38 [CF3]+ |
|
15 |
2.0/4.4 |
2/1 |
492/51 |
473/51 [M-F]+, 373/31 [M-C2F5]+, 295/17 [M-COC3F7]+, 179/100 [M- COC3F7-CO-CF3-F]+, 169/48 [C3F7]+, 119/9 [C2F5]+, 69/34 [CF3]+ |
|
16 |
6.5/- |
3/- |
484/6 |
465/4 [M-F]+, 315/100 [M-C3F7]+, 296/20 [M-C3F7-F]+, 194/31 [M-C3F7-C2F5]+, 145/45 [M-2C3F7]+, 110/12 [M-2C2F5-Cl]+, 169/23 [C3F7]+, 119/5 [C2F5]+, 69/30 [CF3]+ |
|
17 |
5.3/8.0 |
1/1 |
652/12 |
633/6 [M-F]+, 533/3 [M-C2F5]+, 483/11 [M-C3F7]+, 363/5 [M-C3F7-HC2F5]+, 314/0.2 [M-2C3F7]+, 194/25 [M-2C3F7-HC2F5]+, 169/100 [C3F7]+, 119/5 [C2F5]+, 69/26 [CF3]+ |
|
18 |
-/1.9 |
-/1 |
668/4 |
667/2 [M-F]+, 567/1 [M-C2F5]+, 517/3 [M-C3F7]+, 397/1.5 [M-C3F7-HC2F5]+, 348/3 [M-2C3F7]+, 313/3 [M-2C3F7-Cl]+, 229/14 [M-2C3F7-C2F5]+, 169/100 [C3F7]+, 119/4 [C2F5]+, 69/26 [CF3]+ |
|
19 |
2.5/- |
2/- |
688/5 |
669/2 [M-F]+, 569/0.1 [M-C2F5]+, 519/37 [M-C3F7]+, 350/3 [M-2C3F7]+, 315/10 [M-2C3F7-Cl]+, 231/2 [M-2C3F7-C2F5]+, 169/100 [C3F7]+, 119/10 [C2F5]+, 69/43[CF3]+ |
|
20 |
1.6/- |
1/- |
558/28 |
539/5 [M-F]+, 519/3 [M-HF2]+, 439/100 [M-C2F5]+, 320/22 [M-2C2F5]+, 234/12 [M-2C2F5-HCl-CF2]+, 169/3 [C3F7]+, 119/1 [C2F5]+, 69/4 [CF3]+ |
|
21 |
0.6/- |
1/- |
424/48 |
405/4 [M-F]+, 305/100 [M-C2F5]+, 269/5 [M-C2F5-Cl]+, 235/34 [M-C2F5-2Cl]+, 199/9 [M-C2F5-2Cl-HCl]+, 119/6 [C2F5]+, 69/4 [CF3]+ |
|
22 |
-/0.3 |
-/1 |
406/100 |
287/6 [M-C2F5]+, 267/94 [M-C2F5-HF]+, 232/22 [M-C2F5-HF-Cl]+, 169/14 [C3F7]+, 119/6 [C2F5]+, 69/3 [CF3]+ |
|
23 |
0.4/- |
1/- |
574/26 |
555/6 [M-F]+, 455/100 [M-C2F5]+, 419/2 [M-C2F5-HCl]+, 251/8 [M-C2F5-Cl-C3F7]+, 169/22 [C3F7]+, 119/8 [C2F5]+, 69/6 [CF3]+ |
|
24 |
-/0.4 |
-/1 |
422/100 |
284/8 [M-C2F5-H2O]+, 250/6 [M-C2F5-H2O-Cl]+, 169/34 [C3F7]+, 119/16 [C2F5]+, 69/61 [CF3]+ |
|
25 |
8.3/- |
5/- |
594/26 |
575/4 [M-F]+, 555/1 [M-HF2]+, 425/100 [M-C3F7]+, 389/28 [M-C3F7-HCl]+, 363/66 [M-C3F7-HCl-Cl]+, 234/92 [M-C3F7-2HCl-C2F5]+, 169/5 [C3F7]+, 119/12 [C2F5]+, 69/18 [CF3]+ |
|
26 |
-/0.5 |
-/1 |
404/45 |
385/3 [M-F]+, 285/100 [M-C2F5]+, 186/11 [M-C2F5-2Cl-HCO]+, 169/6 [C3F7]+, 119/1 [C2F5]+, 69/14 [CF3]+ |
|
27 |
-/0.3 |
-/1 |
572/59 |
453/100 [M-C2F5]+, 334/20 [M-2C2F5]+, 248/8 [M-2C2F5-Cl-CF2]+, 169/3 [C3F7]+, 119/1 [C2F5]+, 69/8 [CF3]+ |
|
Total: 77.1/71.4 |
||||
|
Compounds based on PCB-Cl |
||||
|
28 |
2.3 |
2 |
356/29 |
337/2 [M-F]+, 237/100 [M-C2F5]+, 201/18 [M-C2F5-HCl]+, 169/1 [C3F7]+, 119/5 [C2F5]+, 69/5 [CF3]+ |
|
29 |
45.4 |
10 |
524/20 |
505/5 [M-F]+, 485/1 [M-HF2]+, 405/100 [M-C2F5]+, 369/1 [M-C2F5-HCl]+ 286/31 [M-2C2F5]+, 250/4 [M-2C2F5-HCl]+, 201/9 [M-2C2F5-HCl-CF2]+, 119/1 [C2F5]+, 69/1 [CF3]+ |
|
30 |
12.9 |
8 |
692/11 |
673/5 [M-F]+, 653/1 [M-HF2]+, 573/100 [M-C2F5]+, 454/7 [M-2C2F5]+, 419/5 [M-2C2F5-HCl]+, 300/9 [M-3C2F5-HCl]+, 169/2 [C3F7]+, 119/1 [C2F5]+, 69/3 [CF3]+ |
|
31 |
4.1 |
2 |
658/11 |
639/5 [M-F]+, 619/1 [M-HF2]+, 539/100 [M-C2F5]+, 370/1 [M-C3F7-C2F5]+, 301/5 [M-2C3F7]+, 300/9 [M-2C3F7-C2F5]+, 169/2 [C3F7]+, 119/1 [C2F5]+, 69/6 [CF3]+ |
|
32 |
1.3 |
3 |
826/13 |
807/12 [M-F]+, 787/3 [M-HF2]+, 707/100 [M-C2F5]+, 419/7 [M-3C2F5-CF3]+, 169/52 [C3F7]+, 119/8 [C2F5]+, 69/38 [CF3]+ |
|
33 |
3.7 |
2 |
694/26 |
675/15 [M-F]+, 525/100 [M-C3F7]+, 371/85 [M-C3F7-C2F5-Cl]+, 201/20 [M-2C3F7-C2F5-HCl]+, 169/26 [C3F7]+, 119/8 [C2F5]+, 69/44 [CF3]+ |
|
34 |
9.3 |
4 |
862/20 |
743/11 [M-C2F5]+, 693/72 [M-C3F7]+, 573/25 [M-C2F5-C3F7]+, 405/100 [M-C2F5-2C3F7]+, 385/56 [M-C2F5-2C3F7-HF]+, 351/11 [M-C2F5-2C3F7-F-Cl]+, 169/82 [C3F7]+, 119/19 [C2F5]+, 69/86 [CF3]+ |
|
35 |
1.4 |
2 |
702/18 |
683/6 [M-F]+, 663/2 [M-HF2]+, 583/100 [M-C2F5]+, 386/1 [M-C2F5-COC3F7]+, 169/17 [C3F7]+, 119/3 [C2F5]+, 69/14 [CF3]+ |
|
Total: 80.4 |
||||
|
Compounds based on PCB-Cl2 (with two chlorine atoms in one aromatic ring/ with two chlorine atoms, one of them in each aromatic ring) |
||||
|
36 |
20.4/12.3 |
5/7 |
390/57 |
371/3 [M-F]+, 351/2 [M-HF2]+, 271/100 [M-C2F5]+, 235/5 [M-C2F5-HCl]+ 201/20 [M-C2F5-2Cl]+, 150/5 [M-2C2F5-HCl-CF2]+, 119/1 [C2F5]+, 69/3 [CF3]+ |
|
37 |
1.7/10.9 |
3/8 |
558/25 |
similar to fragmentation of compound 20 |
|
38 |
6.0/- |
7/- |
574/18 |
similar to fragmentation of compound 23 |
|
39 |
4.6/- |
6/- |
742/25 |
723/3 [M-F]+, 703/2 [M-HF2]+, 623/100 [M-C2F5]+, 457/5 [M-C2F5-F-CO]+, 169/12 [C3F7]+, 119/6 [C2F5]+, 69/40 [CF3]+ |
|
40 |
5.6/- |
4/- |
540/100 |
501/11 [M-HF2]+, 401/79 [M-C2F5-HF]+, 219/45 [M-C2F5-HF-Cl-CO]+, 169/9 [C3F7]+, 119/7 [C2F5]+, 69/15 [CF3]+ |
|
41 |
6.1/- |
7/- |
708/11 |
689/4 [M-F]+, 589/30 [M-C2F5]+, 372/100 [M-2C2F5-CF2-HCO-F]+ 301/12 [M-3C2F5-CF2]+, 169/13 [C3F7]+, 119/5 [C2F5]+, 69/26 [CF3]+ |
|
42 |
2.3/- |
4/- |
406/100 |
similar to fragmentation of compound 22 |
|
43 |
18.5/7.3 |
4/4 |
400/93 |
203/100 [M-COC3F7]+, 175/37 [M-COC3F7-CO]+, 139/37 [M-COC3F7-CO-Cl]+, 115/6 [M-COC3F7-CO-Cl-C2H2]+, 169/13 [C3F7]+, 119/2 [C2F5]+, 69/30 [CF3]+ |
|
44 |
10.8/15.6 |
5/10 |
568/32 |
549/3 [M-F]+, 449/100 [M-C2F5]+, 371/5 [M-COC3F7]+, 343/2 [M-COC3F7-CO]+, 252/1 [M-C2F5-COC3F7]+, 224/26 [M-C2F5-COC3F7-CO]+, 201/9 [M-C2F5-COC3F7-CO-Cl]+, 169/10 [C3F7]+, 119/2 [C2F5]+, 69/10 [CF3]+ |
|
45 |
1.5/- |
1/- |
560/38 |
541/7 [M-F]+, 441/4 [M-C2F5]+, 391/100 [M-C3F7]+, 355/36 [M-C3F7-HCl]+, 320/6 [M-C3F7-2HCl]+, 222/78 [M-2C3F7]+, 152/35 [M-2C3F7-2HCl]+, 169/6 [C3F7]+, 119/5 [C2F5]+, 69/27 [CF3]+ |
|
46 |
0.6/2.6 |
1/2 |
728/38 |
709/6 [M-F]+, 689/2 [M-HF2]+, 559/100 [M-C3F7]+, 390/50 [M-2C3F7]+, 335/20 [M-2C3F7-C2F5-HCl]+, 169/24 [C3F7]+, 119/12 [C2F5]+, 69/38 [CF3]+ |
|
47 |
-/1.5 |
-/1 |
692/11 |
similar to fragmentation of compounds 30 |
|
48 |
-/3.5 |
-/2 |
876/7 |
857/1 [M-F]+, 757/48 [M-C2F5]+, 617/100 [M-2C2F5-HF]+, 469/18 [M-3C2F5-HF-HCO]+, 169/61 [C3F7]+, 119/21 [C2F5]+, 69/76 [CF3]+ |
|
49 |
-/0.9 |
-/2 |
736/45 |
717/9 [M-F]+, 567/12 [M-C3F7]+, 539/100 [M-COC3F7]+, 251/32 [M- COC3F7-2C2F5-CF2]+, 169/60 [C3F7]+, 119/15 [C2F5]+, 69/70 [CF3]+ |
|
50 |
-/0.9 |
-/1 |
862/20 |
similar to fragmentation of compounds 34 |
|
51 |
-/10.1 |
-/8 |
896/54 |
877/17 [M-F]+, 857/3 [M-HF2]+, 777/8 [M-C2F5]+, 707/100 [M-C3F7-HF]+ 553/34 [M-C3F7-HF-C2F5-HCl]+, 169/55 [C3F7]+, 119/11 [C2F5]+, 69/86 [CF3]+ |
|
Total: 78.3/65.6 |
||||
References
- Watts R.J., Teel A.L. // Pract. Period. Hazard. Toxic Radioact. Waste Manage. 2006. Vol. 10. P. 2-9.
- Neta P., Madhavan V., Zemel H., Fessenden R.W. // Amer. Chem. Soc. 1977. Vol. 99. P. 163-164.
- Eberhardt M.K., Fuentes-Aponte A. // J. Org. Chem. 1983. Vol. 48. P. 3444-3448.
- Minisci F., Citterio A. // Acc. Chem. Res. 1983. Vol. 16. P. 27-32.
- Rosso J.A., Allegretti P.E., Mártire D.O., Gonzalez M.C. // J. Chem. Org., Perkin Trans.1999. N 2. P. 205-210.
- Liang C., Bruell C.J., Marley M.C., Sperry K.L. // Chemosphere. 2004. Vol. 55. P. 1225-1233.
- Liang C., Wang Z.-S., Bruell C.J. // Chemosphere. 2007. Vol. 66. P. 106-113.
- Anipsitakis G.P., Dionysiou D.D., Gonzalez M.A. // Еnv. Sci Tech. 2006. Vol. 40. P. 1000-1007.
- Rastogi A., Al-Abed S.R., A Dionysiou D.D. // Applied Cat. B: Env. 2009. Vol. 85. P. 171-179.
- Dogliotti L., Hayon E. // J. Phys. Chem. 1967. Vol. 71. P. 2511-2516.
- Gonzalez M.C., Martire D.O. // Water Sci. Tech.. 1997. Vol. 35. N 4. P. 49-55.
- Madhavan V., Levanon H., Neta P. // Radiat. Res. 1978. Vol. 76. N 1. P. 15-22.
- Serguchev U.A., Beletskaya I.P. // Russ. Chem. Rev. 1980. Vol. 69. P. 2257-2285.
- Tanner D.D., Osman S.A.A. // J. Org. Chem. 1987. Vol. 52. P. 4689-4693.
- Serguchev Yu.A., Davydova V.G. // Zh. Org. Khim. 1982. Vol. 18. N 12. P. 2610-2611.
- Mullin M.D., Pochini C.M., McCrindle S., Romkes M., Safe S.H., Safe L.M. // Environ. Sci. Technol. 1984. Vol. 18. N. 6. P. 468-476.
- Becker, H., Berger, W., Domschke, G., Fanghänel, E., Faust, J., Fischer, M., Gents, F., Gewald, K., Gluch, R., Mayer, R., Müller, K., Pavel, D., Schmidt, H., Schollberg, K., Schwetlick, K., Seiler, E., Zeppenfeld, G. Organikum. Organisch-chemisches Grundpraktikum, sixth ed. VEB Deuutcher Verlag der Wissenschaften, Berlin, 1967. S. 516.
- Metelitsa D.I. // Russ. Chem. Rev. 1971. Vol. 40. P. 563 - 579.
- Eberhardt M.K. // J. Amer. Chem. Soc. 1981. Vol. 103. P. 3876-3878.
- Rosso J.A., Bertolotti S.G., Braun A.M., Mártire D.O., Gonzalez M.C. // J. Phys. Org. Chem. 2001. Vol. 14. P. 300-309.
- Timperley C.M., White W.E. // J. Fluor. Chem. 2003. Vol. 123. P. 65-70.
- Taskinen E. // Structural Chem. 2000. Vol. 11. N 5. P. 293-301.
- Cioslowski J., Liu G., Moncrieff D. // J. Phys. Chem. A. 1997. Vol. 101. N 5. P. 957-960.
- Alegre M.L., Geronés M., Rosso J.A., Bertolotti S.G., Braun A.M., Mártire D.O., Gonzalez M.C. // J. Phys. Chem. A. 2000. Vol. 104. P. 3117-3125.
- Mártire D.O., Rosso J.A., Bertolotti S.G., Le Roux G.C., Braun A.M., Gonzalez M.C. // J. Phys. Chem. A. 2001. Vol. 105. P. 5385-5392.
- Gorbunova T.I., Saloutin V.I., Chupakhin O.N. // Russian Chemical Reviews. 2010. Vol. 79. N 6. P. 511-530.
- Ballschmiter K., Zell M. // Fresenius` J. Anal. Chem. 1980. Vol. 302. N 1. P. 20-31.
- Hetflejš J., Czakóvá M., Řeřicha R., Včelák J. // Chemosphere. 2001. Vol. 44. P. 1521-1529.
- Yang H., Yu H.-X., Han Q.-G., Wang L.-S., Zhang Z. // Chemosphere. 1997. Vol. 35. P. 2657-2663.
- Hagenaars A., Vergauwen L., De Coen W., Knapen D. // Chemosphere. 2001. Vol. 82. P. 764-772.
- Shi J., Liu H., Sun L., Hou H., Xu Y., Wang Z. // Chemosphere. 2011. Vol. 84. P. 296-304.
- Boltes K., Rosal R., Garćia-Calvo E. // Chemosphere. 2012. Vol. 86. P. 24-29.
- Reinscheid U.M., Vervoort L., Zuilhof H. // Chemosphere. 2006. Vol. 65. P. 318-323.
- Hori H., Murayama M., Inoue N., Ishida K., Kutsuna S. // Catal. Today. 2010. Vol. 151. P. 131-136.
- Park H. // Rev. Roum. Chim. 2010. Vol. 55. N 1. P. 611-619.
Acknowledgements
This research was supported financially by the Ural Division of Russian Academy of Sciences (project no 12-M-34-2036).
Recommended for publication by Prof. A. Y. Zapevalov
Fluorine Notes, 2012, 83, 1-2
