Received: June, 2011
Fluorine Notes, 2012, 82, 1-2
Conversion of some perfluoroolefins under the action of nucleophiles as a waste disposal method for industrial fluoroorganic materials
V.V. Bardin
N.N. Vorozhtsov Novosibirsk Institute of Organic Chemistry of the Siberian Branch of Russian
Academy of Sciences, Novosibirsk, Russia
E-mail: bardin@nioch.nsc.ru
Abstract: The methods of the conversion of some by-products of fluoroorganic industry (perfluorinated alkenes, cycloalkenes and azaalkenes) to practically useful substances (polyfluorinated ethers, amines, and heterocycles with fluorinated substituents) were reviewed.
Keywords: Perfluoroalkenes. Nucleophilic substitution. Heterocyclic substances. Perfluorinated alkyl substituents.
Contents
1. Introduction
2. Reactions of nucleophilic substitution and
addition
3. Synthesis of heterocyclic substances with perfluoroalkyl substituents
1. Introduction
Industrial manufacture of perfluorinated polymer materials, greases, technical liquids, etc. entails the formation of liquid wastes, mainly low-molecular oligomers. Such by-products find no application, and they are difficultly disposed by usual methods. However, they may be a feedstock in the manufacture of some other useful fluoroorganic products. One of the possible routes of their utilization consists in reaction with nucleophiles resulting in partially fluorinated substances, followed by exhausting fluorination of the latter by chemical or electrochemical methods. The related target product yields exceed 70-80%, while direct fluorination of hydrocarbon feedstock rarely results in 10-15% yields [1].
Another route of disposal is conversion of above by-products to heterocyclic substances with perfluorinated alkyl chains. Then these products can be used as a feedstock in further fluorination to produce perfluorinated saturated heterocycles with nitrogen and/or oxygen atoms as well as row material for the manufacture of some medical, veterinary, and agricultural substances [2-6]. Because of industrial, ecological, and, undoubtedly, commercial interest, numerous scientific articles and patents are published in this field. This review covers only the most typical processes with simple initial substances, and may be considered as an introduction to the field. The dimers of perfluoropropylene (CF3)2CFCF=CFCF3, (CF3)2C=CFC2F5, perfluoroalkoxyalkenes C3F7OCF=CF2 and C3F7OCF(CF3)CF2OCF=CF2 (derivatives of dimers and trimers of perfluoropropylene oxide), perfluoro-1-ethylcyclohexene (waste in Chromine manufacture) and C4F9N=CFC3F7 (manufactured from perfluorotributylamine) are the objects of this review.
2. Reactions of nucleophilic substitution and addition
Perfluoroacyl fluorides C3F7O[CF(CF3)CF2O]nCF(CF3)COF are oligomers which produced in small amount on polymerization of perfluoropropylene oxide. Heating of their solution in a polar aprotic solvent (diglyme or triglyme) with Na2CO3 results in perfluorinated alkoxyethenes C3F7O[CF(CF3)CF2O]nCF=CF2, easily separated from the reaction mixture. Perfluoroalkoxyethenes C3F7OCF=CF2 (1) and C3F7OCF(CF3)CF2OCF=CF2 (2) react with aliphatic alcohols ROH in THF, dioxane, acetonitrile or THF/ROH mixture in the presence of base to form alkyl polyfluoroalkyl ethers. The approapriate base is KOH or related sodium alcoholate, but triethylamine proved to be non-effective.
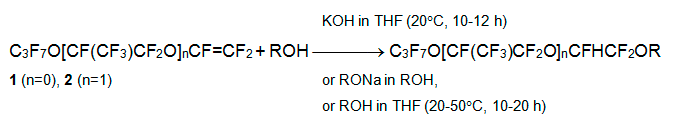
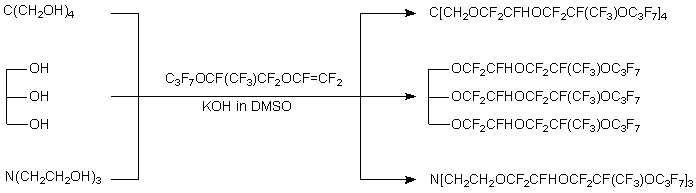
Aliphaticalcohols of normal constitution (R = CH3,C2H5,C3H7,C4H9), isopropyl- or isobutyl alcohols, methyl- or ethyl- cellosolves oreven low-nucleophilic fluorinated alcohols (CF3CH2OH,C6F5CH2CH2OH and C6F5OCH2CH2OH) react in this way [7, 8]. Phenols, 4-R'C6H4OH(R' = CH3OCH2CH2,CH3OC(O)CH2CH2) or C6F5OH, form similar products. When reagent contains several hydroxy groups(polyols etc.), all of them are involved into addition process insequence. Series of polyfluorinated dialkyl ethers were producedfrom polyatomic alcohols (ethylene glycol, triethylene glycol, glycerine, pentaerythritol), triethanolamine or S(CH2CH2OH)2[7-9].
For comparison, reaction of perfluoropropylene with alcohols ROH (R =Me, Et, Pri,CHF2CF2CH2) in the presence of KOH or RONa gave polyfluorodialkyl ethers andalkoxypentafluoropropylene CF3CF=CFOR as admixture [10].
Unlike O-nucleophiles, the addition of amines across C=C bond in perfluoroalkoxyethenРµs occurs without any base. Thus, ethers 1 and 2 react with equimolar amount of dialkylamine HNAlk2, pyrrolidine, piperidine or morpholine at 25В°C forming (2-polyfluoroalkoxyethyl)dialkylamines, which can be easily separated by distillation. If the reaction was performed in the presence of triethylamine and the resulted reaction mixture was treated with water, dialkyl(1H-perfluoroalkoxy)acetamides were obtained [9, 11].
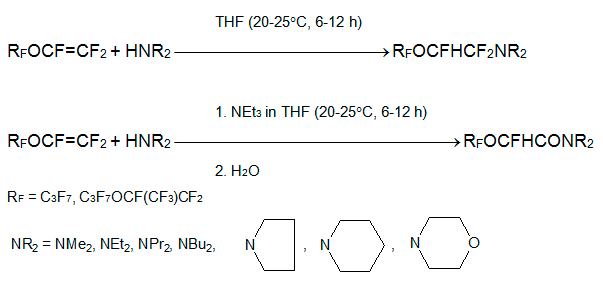
Under the same conditions, imines RFOCFHCF=NAlk (separated by distillation) or alkylamides RFOCFHCONHAlk (after hydrolysis) were produced with good yields from ethers 1 and 2 and primary amines H2NAlk [9, 11].
Unlike terminal perfluoroalkenes (perfluoropropylene, perfluoroalkoxyethenes 1 or 2), the reactivity of internal perfluoroalkenes, R1CF=CFR2or R1R2C=CFR3, depends on cooperative electronic and steric effects of vinylic fluorine atoms as well as perfluoroalkyl groups R1,R2,and R3 at C=C bond. This circumstance determinates the nature of products and their molar ratio in reactions with nucleophiles. The addition of a nucleophile to sp2-hybridised carbon gives carbanion, which further fate depends on the nature of the substituent and properties (basicity, polarity) of solvent. Noteworthy, that nucleophiles can attach to either sp2-hybridised carbon atoms or to neighbouring carbons (transfer of the reaction centre). It results in a diversity of products, which can undergo additional secondary transformations. More details of the reactions of internal perfluoroalkenes were reviewed earlier [12] whereas here we will focus on results of addition (substitution) by O-, N-, S- and P-nucleophiles to selected substrates, perfluoro-2-methyl-2-pentene (3), perfluoro-4-methyl-2-pentene (4), perfluoro-1-ethylcyclohexene (5), and perfluoro-4-aza-5-nonene (6).
Earlier it was reported that the interaction of alkene 3 with methanol in the presence of triethylamine resulted in the replacement of vinylic fluorine with methoxy group, and in the addition of methanol at the multiple bond (the total product yield was 44% at molar ratio 1:1), besides minor isomers 1,3-dimethoxyperfluoro-2-methyl-1-pentenes [13, 14]. Alkene 4 reacts only with sodium methylate to give isomeric 1,3,4-trimethoxyperfluoro-2-methyl-1-pentenes (yield 57%), while the formation of monomethoxy-containing products was not reported [13]. The detailed study of reaction of perfluoroalkenes 3 or 4 with MeOH was performed at various conditions in the effort to prepare raw materials for the manufacture of perfluoro-2-methyl-3-methoxypentane by gas-phase fluorination. The considerable role of solvents (MeOH, DMF, MeCN, with THF or acetone additives), reagent ratio, order of reagent mixing, and the nature of base (MeONa, KOH, NEt3) was revealed. The best conditions and main reaction products are shown below [15].
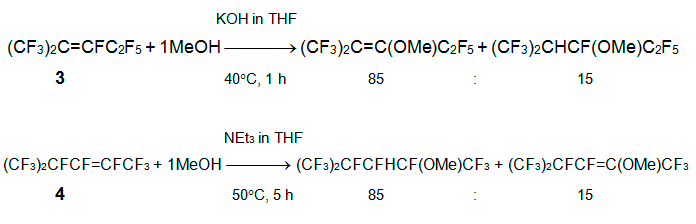
Perfluoro-2-methyl-2-pentene reacts with О±,О±,П‰-trihydroperfluoroalkanols (telomeric alcohols) in the presence of triethylamine or pyridine in MeCN at 70В°C to yield only products of alkoxydefluoridation. Cesium fluoride proved to be the most efficient base that is active efficiently even at 20В°C [16].
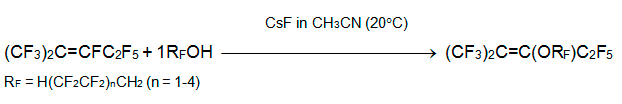
It should be noted that cesium fluoride is efficient agent in the reactions of telomeric alcohols with polyfluoroaromatic substances where polyfluorinated aryl alkyl ethers were produced [16, 17]. The latter were used for feedstock in the manufacture of perfluorodialkyl ethers by gas-phase fluorination along with polyfluorinated alkenyl alkyl and dialkyl ethers.
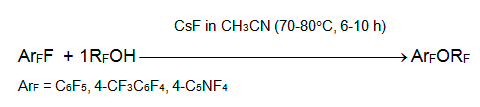
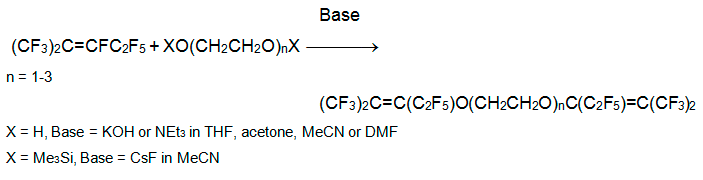
In reaction of perfluoro-2-methyl-2-pentene with mono-, di-, or triethylene glycols (mole ratio 3:1) in acetonitrile (THF, acetone, DMF) in the presence of triethylamine (or KOH), the alkoxide adds to carbon of the multiple bond to form a carbanion. The latter eliminates fluoride anion to form the related partly fluorinated diethers. The usage of 1,2-bis(trimethylsiloxy)ethane instead of pattern diols in the presence of CsF in anhydrous MeCN increases yield of desired products [18].
Reaction of alkene 3 with equimolar (or excess) amount of ethylene glycol in the presence of NEt3 results in a complex mixture of dioxolane or dioxepine derivatives.
Phenol or its derivatives with electron-donating substituents react with perfluoro-2-methyl-2-pentene in THF at 40В°C yielding exclusively 2-aryloxypentafluoro-2-methyl-2-pentenes. The reaction with 2-hydroxybenzimidazole and 2-hydroxybenzothiazole occurs in the same manner [19].
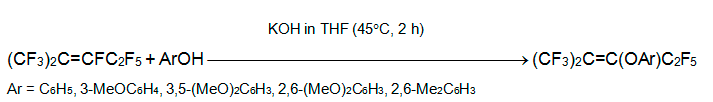
The phenols with electron-withdrawing substituents react with alkene 3 to form 2-aryloxypentafluoro-2-methyl-2-pentene and 1-aryloxypentafluoro-2-methyl-2-pentene. The content of the former varies from a few % (Ar = 3-NO2C6H4, 4-NO2C6H4,3-C5NH4) to 100% (Ar = 2,6-Cl2C6H3,C6F5)[19].
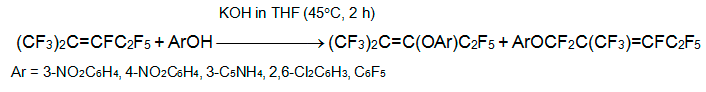
Earlier it was shown that alkene 3 reacts with acetone oxime in the presence of triethylamine to form the products of both vinylic substitution and addition to C=C bond [20]. In contrast, acetophenone oxime gave only product of addition.
Dehydrofluoridation of the latter with triethylamine as well as reaction of 3 with acetophenone oxime in the presence of NEt3 result in formal substitution of fluorine at C=C bond [21].
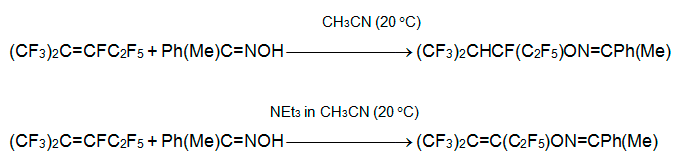
The usage of acetophenone trimethylsiloxyoxime as nucleophile results in a product of addition that may further react with excess of nucleophile by its terminal carbon atom.
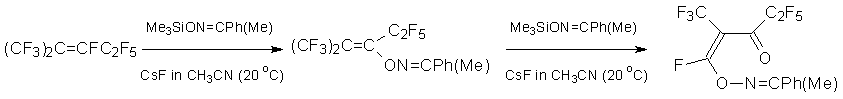
It is interesting that heating of alkene 3 with hexamethyldisiloxane and CsF in DMF results in a silyl enolate that converts to ketone after hydrolysis [22].
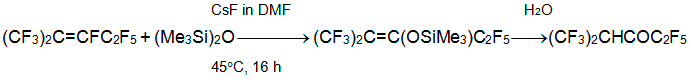
While O-nucleophiles and alkene 3 form products of vinylic fluorine substitution, reactions of N-nucleophiles lead to a diversity of products. Thus, azides react with alkene 3 even at -20В°C to give thermally unstable perfluoro-2-methyl-3-azido-2-pentene [23].
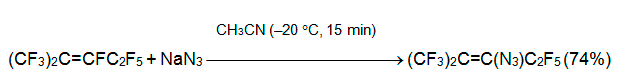
Though ambident thiocyanate anion is a weaker nucleophile, basic additives are not required in this case too. The reactions of sodium (potassium) thiocyanate with an internal perfluoroalkenes 3, 5 or azaalkene 6 in a polar aprotic solvent (acetonitrile, acetone, THF, DMF, sulfolane, DME) at 40-60В°C results always in the vinylic fluorine substitution [24, 25].
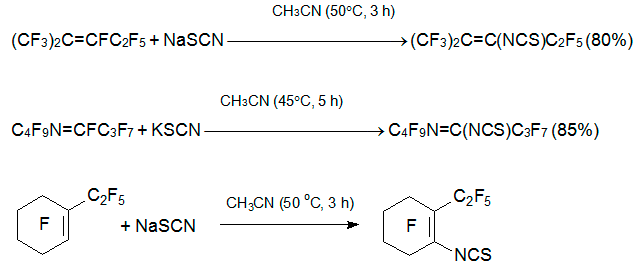
Azoles react with alkene 3 in the presence of NEt3 like O-nucleophiles to give hydrolytically stable enamines [26]. Similar products are produced by reflux of 3 with amide (pyrrolidone, succinimide, phthalimide) in the presence of triethylamine [27].
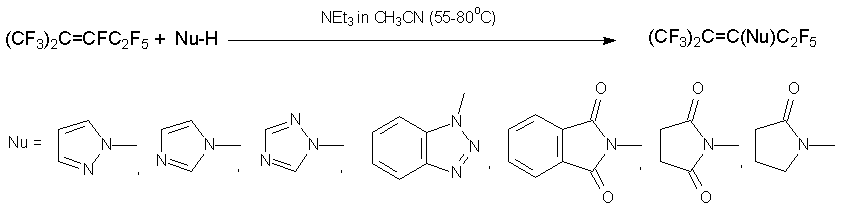
In going to highly basic secondary amines, the alternative reaction route, the substitution of fluorine at position 1, becomes more essential. The reason consists in enriched concentration of highly electrophilic isomer CF2=C(CF3)CF2C2F5, that is in equilibrium with isomers 3 and 4. Thus, the reactions with piperidine, morpholine, or bis(4-heptafluorotolyl)amine result in A:B products in 1:3 molar ratio [27], whereas reaction with dipropylamine, dibutylamine, or diallylamine results in enamine B only [28].
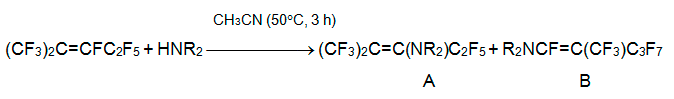
Reaction of alkene 3 with hexamethyldisilazane in the presence of base begins with attack of N-anion at C-3 atom and leads to formation of carbanion [(CF3)2C-CF(C2F5)N(SiMe3)2]-. The further fluoride-catalyzed reactions result in 2H-perfluoroisohexane, bis(perfluoro-2-methylpent-2-en-3-yl)amine, and 2H-3-iminoperfluoro-2-methylpentane. The latter is also the main product of the reaction of alkene 3 and anhydrous NH3 in THF at 0В°C, while at room temperature the further conversions occur [22].
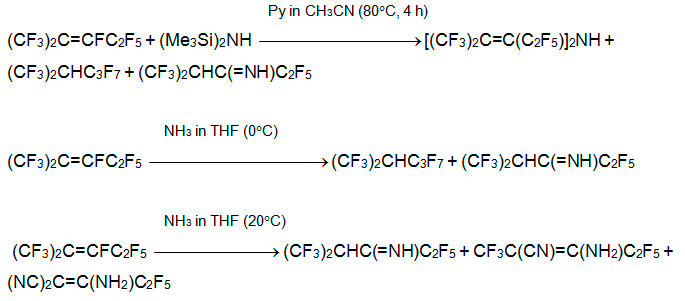
The direction of reactions with primary amines H2NR depends considerably on the nature of R, the ratio alkene/ amine, and the basicity of media. The reaction of alkene 3 with primary alkylamines (including sterically hindered ones) results in imines and admixture (<10%) of azetine and azetidine derivatives (see below) [29-31].
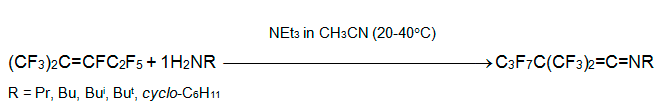
Reaction of triethylphosphite (P-nucleophile) with internal perfluoroalkenes proceeds by quite different route. When alkene 3 is mixed with 2 equivalents of P(OEt)3, fast exothermic reaction occurs to form diethyl perfluoro-2-methylpenta-1,3-diene-3-yl phosphonate. However, in the reaction with perfluoro-1-ethylcyclohexene the related ester is the minor product, while the main product becomes 1-ethylperfluoro-2-ethylcyclohexene. Under the same conditions, perfluoro-5-aza-4-nonene and perfluoro-1-azacyclohexene produce complex mixture [32].
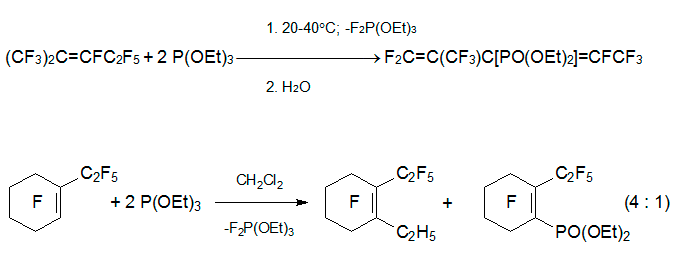
Such diversity in the products of reactions of internal perfluoroalkenes with triethylphosphite with respect to those of O- or N-nucleophiles arises from the relative stability of intermediately formed fluorophosphoranes and specific rearrangements including fluorine atom migration which are typical for them.
3. Synthesis of heterocyclic substances with perfluoroalkyl substituents
In some cases the existence of several pathways for the stabilization of polyfluorinated carbanion formed by addition of N-nucleophile to internal alkene (CF3)2C=CFC2F5, or to azaalkene C4F9N=CFC3F7 , results in nitrogen-containing heterocycles. The treatment of alkene 3 with sodium azide in MeCN/EtOH at -10В°C leads to substituted 1,2,3-triazoline, while the same reaction in MeCN at 20В°C gives mainly substituted azirine [23].
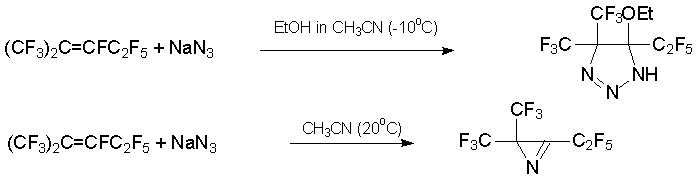
As it has been mentioned above, the interaction of primary amines with alkene 3 at 2:1 ratio results in imines. However, in the presence of triethylamine the main reaction products are substituted 4-alkylimine-2-azetine. Probably, their precursors are 4,4-difluoro-2-azetines that were isolated from the some reaction mixture [29, 30].
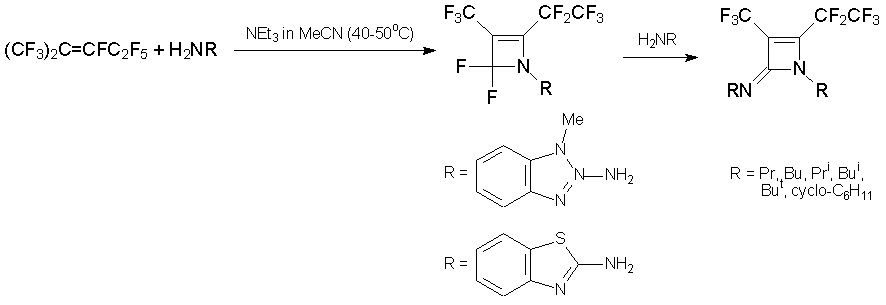
Under similar conditions, azaalkene 6 and primary amines form substituted diazetines and perfluorobutyramide [31, 33].
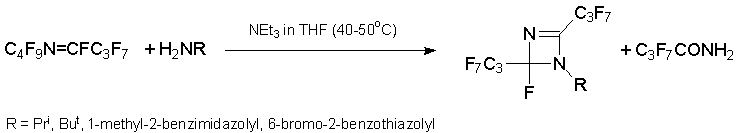
Reaction with anilines should be discussed separately. Treatment of azaalkene 6 with weakly nucleophilic ArNH2 leads to diazetine derivatives (Ar = C6F5,2-NO2C6H4,4-NO2C6H4) or azetine (Ar = 2,6-Cl2C6H3), while reaction with highly nucleophilic anilines (Ar= C6H5,4-MeOC6H4,4-FC6H4,2,6-(CH3)2C6H3) results in 1,2-dihydroquinazaline derivatives. Under the same conditions, alkene 3 gives quinoline derivatives [34].
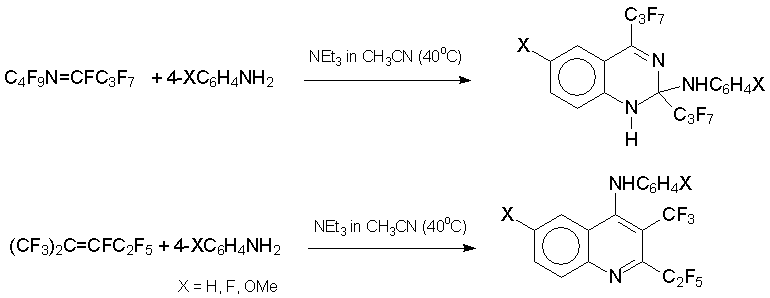
Another path to the formation of perfluoroalkylated heterocycles consists in reaction of internal perfluoroalkenes with bifunctional nucleophiles. A series of oxygen-, nitrogen- or sulfur-containing heterocycles of various sizes containing two or three heteroatoms were prepared from alkene 3 or azaalkene 6, respectively. The method efficiency was exemplified by the synthesis of four-, five-, six-, seven- , and nine-membered heterocyclic systems. All reactions occur in MeCN or THF in the presence of triethylamine at 20-50В°C within 2-5 h. Some typical examples of the synthesis are shown below.
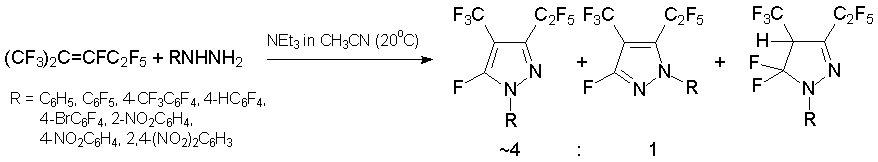
Hydrazine derivatives provide an example of 1N,2N-binucleophile. Arylhydrazines react with alkene 3 to form (trifluoromethyl)(pentafluoroethyl)fluoro-1-arylpyrazoles and small quantity of the corresponding pyrazoline [33, 35, 36].
Under similar conditions, 3,5-bis(heptafluoropropyl)-1,2,4-triazoles were produced from azaalkene 6 in 40-70% yield.
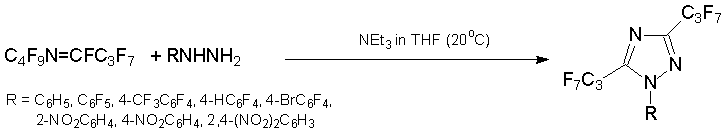
2-Mercaptobenzimidazoles [37], 1,2,4-triazole-3-thiol, and 1,4,5,6-tetrahydropyrimidine-2-thiol [38] are 1N,3S-binucleophiles. Their reaction with 3 allowed obtaining five-membered N,S heterocycles with exo-tetrafluoroethylidene fragment.
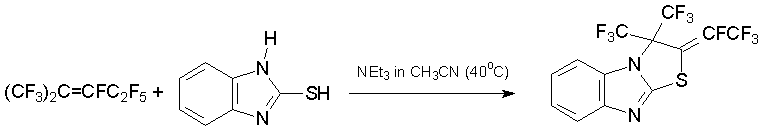
Alternatively, condensation of alkene 3 or azaalkene 6 with 1N,3N-binucleophiles results in six-membered heterocycles which are pyrimidine or 1,3,5-triazine derivatives, respectively. This is exemplified by the production of substituted pyrimidinons from 2-aminobenzothiazole, 2-aminothiazole, 2-amino-5-chlorobenzoxazole [37], urea, benzamidines, or guanidine [39, 40].
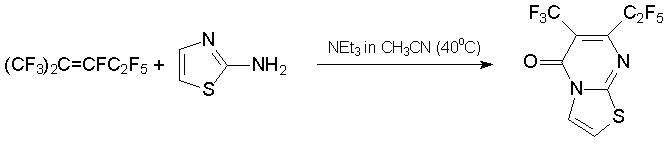
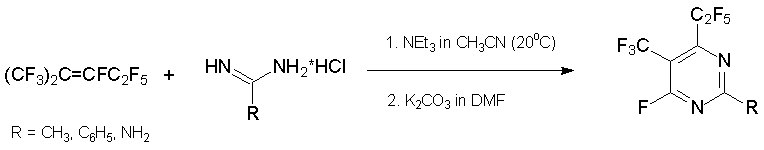
The related reactions with azaalkene 6 result in 2-R-4,6-bis(heptafluoropropyl)-1,3,5-triazine. The use of urea as binucleophile results in substituted 1-hydrooxytriazine that was isolated in keto-form [39, 40].
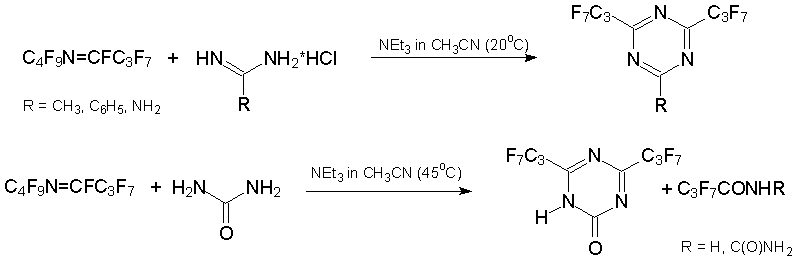
The interaction with 1N,4N-, 1O,4O-, or 1N,4O-binucleophiles results in the formation of seven-membered heterocycles. For example, the dihydrodioxazepine and triazepine derivatives were synthesized from azaalkene 6 and ethylene glycol respectively, while reactions with substituted 2-aminoethanols result in bis(heptafluoropropyl)dihydrooxadiazepines [41].
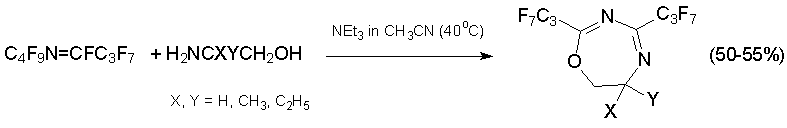
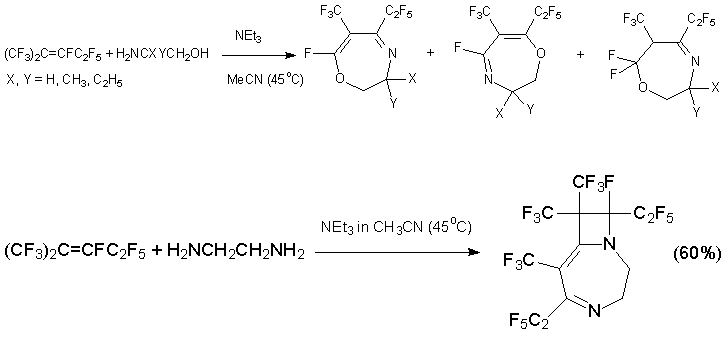
Di- and tetrahydrooxazelines with trifluoromethyl and pentafluoroethyl groups at C=C bond are produced by reaction of alkene 3 with 2-aminoethanol derivatives. Unexpectedly, the usage of 1,2-diaminoethane instead of 2-aminoethanol gave substituted diazabicyclononadiene which is product of sequential reaction of initially formed diazepine with alkene 3 [41, 42].
At the same conditions, diazabicycloalkene of other type is formed from 1,6-hexamethylenediamine (1N,7N-binucleophile) [42].
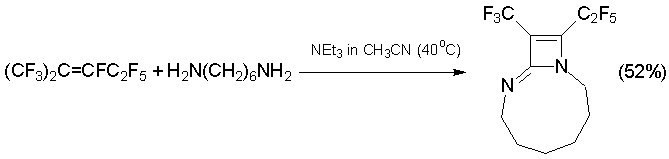
The presented examples describe the preparation of heterocyclic substances, where the initial step is nucleophilic substitution of fluorine at =CF-C moiety of perfluoroalkene (perfluoroazaalkene). However, there is the path to heterocycles, the reaction of nucleophiles with polyfluorinated alkenes containing =C(X)-C moiety, where X is a polyfunctional substituent. It was conveniently illustrated by synthesis of thiazole or thiazine derivatives from perfluoro-2-methyl-3-isothiocyanate-2-pentene (7) and a series of O-, N-, S-, and P-nucleophiles.
Polyfluoroalkene 7 was synthesized with high yield by reaction of perfluorinated 2-methyl-2-pentene 6 and sodium (or potassium) thiocyanate (chapter 2).
O-Nucleophiles (alcohols, phenols) react with alkene 7 in the presence of base to produce esters (CF3)2CHC(C2F5)=NC(S)OR. Under the action of base (K2CO3in DMF, 50-70В°C) they underwent intramolecular cyclization giving 2-alkoxythiazole or thiazolinone derivatives. These heterocycles are produced directly by heating of alkene 7 and ROH in the presence of triethylamine [43, 44].
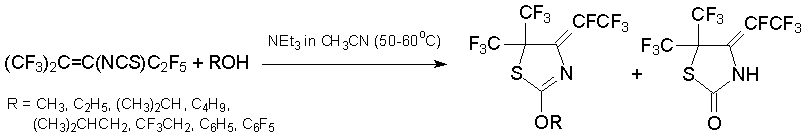
The reaction between alkene 7 and N-nucleophiles proceed ambiguously. Thus, in excess methylamine substituted 3H-pyrimidine-4-thione is produced with high yield [45].
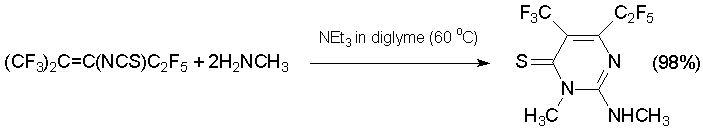
Under the mild conditions, reactions with secondary amines lead to other type of six-membered heterocycles, i.e. to 2-substituted 5-trifluoromethyl-4-pentafluoroethyl-6H-1,3-thiazines [46, 47].
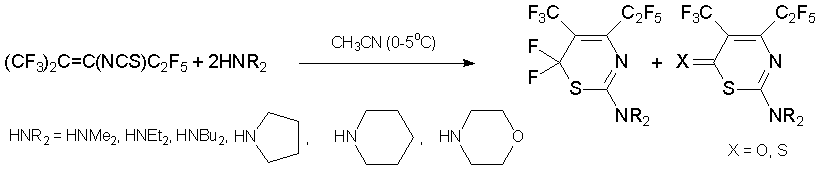
The interaction with azoles in the presence of triethylamine results in the formation of 2-substituted 4,5-dihydrothiazoles in high yields [48, 49]. It should be noted that ambident 1N,3O- and 1N,3S-binucleophiles react with alkene 7 only by nitrogen atom [50].
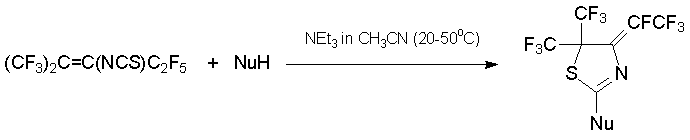
NuH = imidazole, pyrazole, 1,2,4-triazole, benzotriazole, carbazole, phenothiazine, benzimidazole, 2-mercaptobenzoxazole, 2-mercaptothiazole, 2-mercaptopyridine
The results of reaction of monodentate S-nucleophile depend on the strength and nature of base. Base-free reaction of alkene 7 with thiol results in alkyl N-(perfluoro-2-methyl-2H-penta-3-ylidene)dithiocarbamate. The heating of alkene 7 with butanethiol and K2CO3 in DMF gives a mixture of 3-butylthiopentafluoro-2-methyl-2-pentene and substituted dihydrothiazole, while the same procedure with triethylamine in acetonitrile results only in dihydrothiazole derivatives. The same result is obtained in reactions with sodium or potassium thiolates [51, 52].
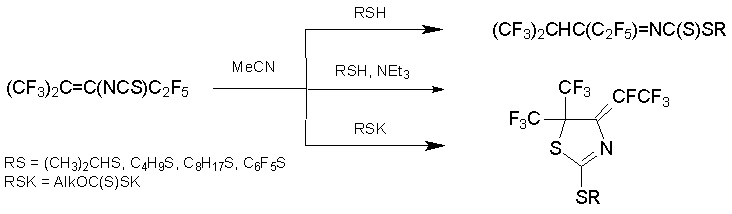
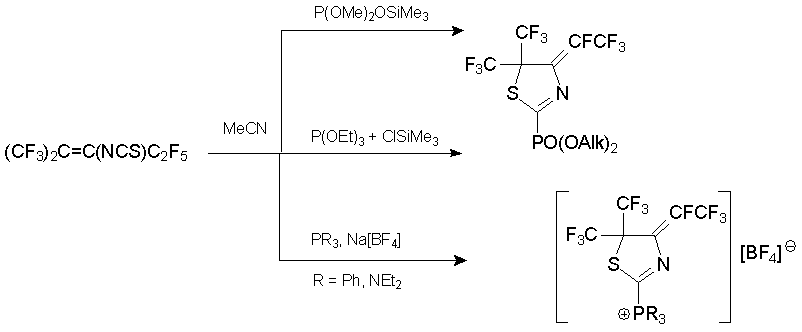
A series of phosphorus-containing dihydrothiazoles was prepared from alkene 7 and P(III)-nucleophiles. In the presence of fluoride anion acceptor, thiazolylphosphoric acid derivatives or phosphonic salts were formed in high yields [53].
References
Recommended for publication by Prof. V.E. Platonov
Fluorine Notes, 2012, 82, 1-2
