Received: December 2011
Fluorine Notes, 2012, 82, 9-10
Particularities of thermolysis for di (polyfluoroalkyl) peroxidicarbonates and theirs application
A.I.Rakhimov, L.A.Butkovskaj
Volgograd State Technical University Russian Federation, 400005, Volgograd, Lenin ave., 28
e-mail: organic@vstu.ru
Abstract: It is shown that thermolysis of di(polyfluoroalkyl)peroxy dicarbonates,[X(CF2)nCH2OC(O)O]2, where X=H, F; n=1, 2, 4, 6 leads to generation of polyfluoroalkoxy-radicls initiating by vinylchloride polymerization. Obtained poly vinylchloride has more thermostability . It is led to lowering of consumption of epoxide soya oil for film receive.
Keywords: di(polyfluoroalkyl)peroxydicarbonates, polyfluoroalkoxy radicals, polyvinylchloride.
It had been proved earlier, that di(polyfluoroalkyl) peroxydicarbonates allowed obtaining the suspension polyvinylchloride of upgraded thermal and light resistance [ 1].
Here we have been considering the kinectics of thermal decomposition of di(polyfluoroalkyl)peroxidicarbonates, [X(CF2)nCH2OC(O)O]2, where X=H, n=2 (I), n=4(II) in benzene and ethylbenzene at the initial peroxide concentration equal to 4·10-2 mole/l. It allows minimizing the processes of induced decomposition with participation of free radicals forming out of peroxide.
In Table 1 is shown the thermolysis rates, half-life periods (τ1/2) and activation energy of mentioned peroxides versus largerly used di(n-butyloxyethyl)peroxydicarbonate (III). Introducing of perfluorinated carbonic chain into di(alkyl)peroxydicarbonate (I) and its lengthening (II) more than twice decreases the thermolysis rate constant in benzene compare to unsubstituted peroxide III: ktherm. at 70°C for I, II and III are respectively equal to 3.30, 3.10 and 7.36 c-1. Half-life period, τ1/2 of peroxides at 70°C alters as follows: I – 35 min, II – 38 min, III – 16 min.
Table 1. Kinectic parameters of thermolysis of di(polyfluoroalkyl)peroxidicarbnates (concentration 4·10-2 mole/l) compare to di (n-butyloxyethyl)peroxidicarbonate (III) (concentration 1·10-2 mole/l)
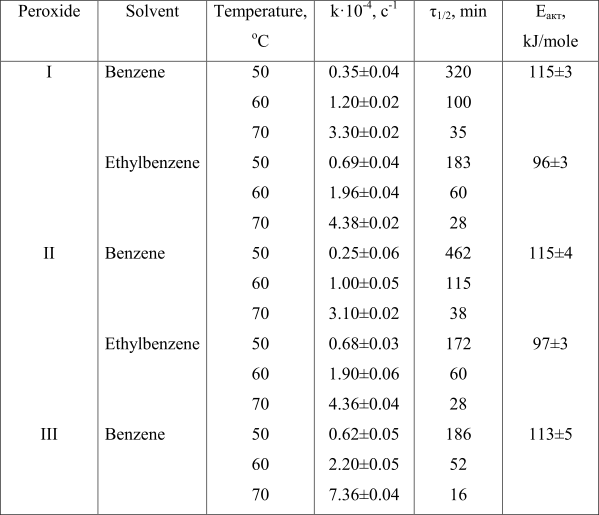
Half-life period, rate constants of monomolecular decomposition and activation energy at transfer from benzene to ethylbenzene differ greatly (Table 1): the thermolysis activation energy drops from 115 to 96–97 kJ/mole. The peroxides’ thermolysis rate constants differ most significantly at 50°C: I 0.35 c-1 (benzene) and 0.69 c-1 (ethylbenzene); II 0.25 c-1 (benzene) and 0.68 c-1 (ethylbenzene). The increase of peroxydicarbonates’ thermolysis rate be observed under transfer from benzene to ethylbenzene for peroxide I almost twice and for peroxide II in 2.7 time (at 50°C). This is explained by reactivity of polyfluoroalkoxyl radicals in tearing off reaction of hydrogen atom from ethylbenzene. Forming during that process phenylethyl radicals are attacking peroxydicarbonate’s peroxide group:
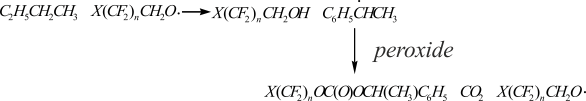
High concentration of 1,1,3-trihydroperfluoropropanol (1,27 mole per 1 mole of peroxide), carbon dioxide (1,58 mole per 1 mole of peroxide) and carbonates (0, 28 moles per 1 mole of peroxide) in the products of peroxide I thermolysis in ethyl benzene at 70°C points out on the formation of polyfluoroalkoxyl radicals.
Forming during monomolecular decomposition of peroxidicarbonate I according to O – O bond carbonate radicals break down forming tetrafluoropropoxy radicals and CO2 (1.58 mole of CO2 per mole of decomposed peroxide). The main part of tetrafluoropropoxy radicals (1.27 mole per 1 mole of decomposed peroxide) reacts with ethylbenzene, which leads to obtaining of 1,1,3-trihydroperfluoropropanole (1.27 mole per 1 mole of peroxide decomposed). The small part of these radicals (0.31 mole per 1 mole of decomposed peroxide) undergoes β-decomposition, which coincides with quantity of formaldehyde found.
Electrophilic properties of polyfluoroalkoxy radicals generated out from polyfluoroalkylperoxydicarbonate during polymerization of vinyl chloride make ease the attack of H2C-group of vinyl chloride compare to unsubstituted alkoxy radicals generated out from di(alkyl) peroxydicarbonates and promote polymerization of vinyl chloride. That decreases the defects in macromolecular chain of polyvinylchloride (effect of nanomodification):
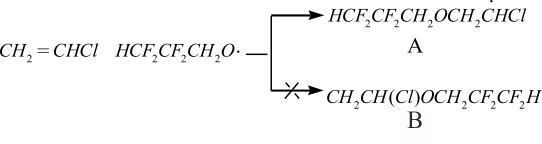
Radical A is more stable than radical B (on 0.310 eV). Therefore radical A is more preferable. The suspension polyvinyl chloride obtained with use of di(polyfluoroalkyl)peroxydicarbonates has more ordered structure and keeping of labil groups is smaller. That’s why nanomodificatid polyvinylchloride is significantly more stable, than during the applying of di ( /em>-butyloxyethyl)peroxydicarbonates [2].
Parameters data on vinyl chloride polymerization process over di(polyfluoroalkyl)peroxydicarbonates as initiators and properties description of obtained polyvinylchloride samples are shown in Table 2. In the same Table you will find comparative data, which characterize polymer obtained over commercially used unsubtituted peroxydicarbonate - di(n-butyloxyethyl)peroxydicatbonate.
Polyvinyl chloride obtained with fluorine containing initiators is characterized by its 3,0 -3,5 times bigger thermal resistance compare to PVC, which had been obtained using unsubstituted peroxydicabonate as an initiator under analogous conditions, which can be explained by introduction of initiator’s fluorinated fragments into polymer chain. The increasing of thermal stability is seen as well in the lowering of its dehydrochlorination rate (Table 2) [3].
Table 2. Parameters of suspension polymerization of vinyl chloride with use of di(polyfluoroalkyl)peroxidicarbonates compare to di(n-butyloxyethyl)peroxidicarbonates (III) and polymer properties
|
Initiator |
t °c |
Period, |
Conversion, % |
Êef |
Iirreversible adsorbtion of plasticizer,% |
poured |
Dehydrochlorination rate, V·107 |
Beginning time of colour change to rolling, min |
|
I |
53 |
6 |
82 |
71 |
21 |
0,50 |
4,3 |
30 |
|
II |
53 |
5 |
81 |
71 |
25 |
0,49 |
4,1 |
35 |
|
III |
53 |
7 |
82 |
72 |
20 |
0,50 |
5,5 |
10 |
It is also necessary to mention, that obtained with new initiators polyvinyl chloride samples are characterized by even distribution of particles according to their size. Polymer particles are homogeneous by their composition, their form is spherical and surface is highly developed. Particles have porous structure. Formative particles have also higher (on 5 – 25%) adsorption rate of plasticizer and better adsorbability (much more mass of adsorbable plasticizer). High thermal stability of obtained PVC is major problem in manufacture of PVC compositions.
Presented in Table 3 properties of PVC – compositions obtained during processing of such polymer confirm that conclusions.
Table 3. Properties of compositions based on polyvinyl chloride S-7058 Ì
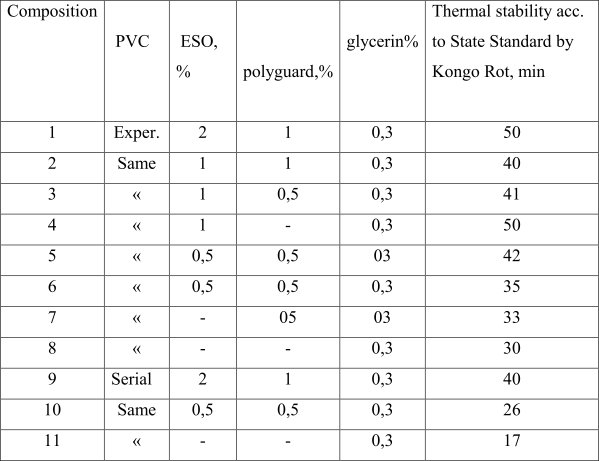
The use of obtained PVC over di(polyfluoroalkyl)peroxidicarbonates (Table 3) allows decreasing of consumption of epoxidesoya oil (ESO) in 2-4 times and eliminating the necessity to use polyguard (composition 4 and 5), nevertheless the thermal stability of film formed at that exceeds or stays at the level of film obtained from serial polymer according to standard recipe (composition 9). The samples of films had been prepared by rolling of PVC-compositions in 10 minutes time at temperature of 170 °C and clirience between rolles equal to 0,6 mm.
EXPERIMENTAL PART
The kinetics of thermal decomposition of di(polyfluoroalkyl)peroxidicarbonates has been studied by ampule method in benzene and ethylbenzene in the atmosphere of purified nitrogen at temperatures of 50, 60 and 70°C in ultrathermostate. Ampoules (volume is equal to 1ml) were taken out (2 parallel samples) in certain period of time cooled and after opening using method of iodidemetric titration the quantity of nondisintegrated peroxide was estimated [1].
Studying of products’ composition of peroxidicarbonates thermolysis in ethylbenzene solution was carried out after keeping samples at 70°C for not less than 10 periods of half-life in vials of 15 ml each. Gasiform products were analyzed at chromatograph «Colour – 104», stainless steel column 300×0.4 cm, sorbent – activated charcoal AG – 3. Column temperature - 227°C, detector – 250°C, evaporator – 300°C. Gas-carrier – helium, pressure 25 kgf/cm2. Qualitative composition of gases was determined by comparing the keeping periods of pure samples and components of the reaction mixture, the amount was estimated using method of simple norming.
Liquid products of the reaction mixture were identified and estimated using chromatograph «Colour – 134», column 300×0.4 cm, adsorbent – SE-30 (5%) at chromatone N-AW. Column temperature 60°C, detector’s t – 110°C, evaporator’s t – 270°C. Gas-carrier – helium had been fed into at a rate of 20 cm3/min, the rate of diagram’s movement was 600 mm/h. The indentification of liquid products was carried out using method of “witnesses” by comparing keeping periods of pure samples, their perfect samples in ethylbenzene and components of reaction mixture. Quantitative measurements were carried out using method of inner standard [4].
Suspension polymerization of vinyl chloride was carried out in steel autoclave of capacity equal to 4,0 L, equipped with mixer (350 rotations/min) and automatically controlled cooling-heating system. 2000ml of desalted water, 2,0g of sodium hydroxide, 1,2g of methyl cellulose mark F -65 are put into the autoclave. The content of autoclave is vacuummized, after that 1000g of vinyl chloride containing initiator in amount of 0.01 – 0.1% of monomer weight are loaded inside. The temperature of polymerization is 53-54 °C. The ending of process is at pressure falling by 2 atm. The vinyl chloride polymerization process parameters were estimated using known ratios [5], the properties of polymer obtained are listed in Table 2.
The thermal stability of polyvinyl chloride was estimated according to the rate of hydrogen chloride elimination from PVC during thermal destruction in vacuum. The method is based on quantitative determination/ estimation of isolating hydrogen chloride by mercurymetrical titration.
Thus, the application of di(polyfluoroalkyl)peroxidicarbonates during polymerization of vinyl chloride allows significant (by 3,5 time) increasing of thermal stability of polyvinyl chloride obtained and simplifying of its processing technology.
References
[1]. Rakhimov A.I., Butkovskaj L.A., Baklanov A.V. // News of VStTech.U. «Chemistry and Technology elementoorganic monomers and polymeric materials». Volgograd. 2008. N 1. p. 85.
[2]. Rakhimov A.I. Initiators for Manufacture of PVC. New York: Nova Science Publishers Inc., 2008. 182 p.
[3]. Rakhimov A.I. Chemistry and Technology of Organic Peroxide Compounds. Moscow: Chemistry, 1979. 389 p.
[4]. Rakhimov A.I., Butkovskaj L.A.,Baklanov A.V. Zegelmaní V.I. Method of Suspension of PVC Synthesis. Patent USSR. 1431302,1987.
[5]. Antonovskii V.L., Buzlanova M.M. Analitic Chemistry of Organic Peroxide Compounds. Moscow: Chemistry. 1978. 308 p.
[6]. Oudian D.. Found Chemistry Polymers. – Moscow: Mir. 1974. 614 p.
Recommended for publication by Prof. Alexander I. Rakhimov
Fluorine Notes, 2012, 82, 9-10
