Received: May, 2012
Fluorine Notes, 2012, 82, 7-8
RESEARCH OF THE REACTION OF DIETHYL CHLOROMALONATE WITH POTASSIUM FLUORIDE IN APROTONIC SOLVENTS
O.B. Sambueva 1,2, V.A.Yanovsky 1, N.F.Mingalimov 1, A. N.Tretyakov 1, O.S.Andrienko 1,2, V.I.Sachkov 1,3
1) Siberian Physico-technical Institute named of the academician V.D. Kuznetsov of
Tomsk State University, 634050, Russia, Tomsk, Novosobornaya sq.1, tel. +7(3822)412319,
e-mail: itc@spti.tsu.ru
2) Institute of Atmospheric Optics named of V.E. Zuev Russian Academy of Sciences, Siberian Branch, 6340211, Russia, Tomsk, Academician Zuev sq. 1
3) Institute for Problems of Chemical and Energetic Technologies Russian Academy of Sciences, Siberian Branch, 659322, Russia, Biysk, st. Socialisticheskaya, 1
Abstract: The reaction of diethyl 2-chloromalonate with potassium fluoride in aprotonic solvents has been studied as a perspective method for producing diethyl 2-fluoromalonate. The influence of the reaction conditions on the composition of the reaction products has been investigated. Using quantum-chemical methods the energy barrier of the reaction has been calculated as function of the dielectric constant of the solvent. It has been established that the introduction of catalyst 18-crown-6 in the system increases the concentration of diethyl-2-fluoromalonate to 50%.
Keywords: diethyl 2-fluoromalonate, diethyl 2-chloromalonate, potassium fluoride.
The practical importance of diethyl fluoromalonate (DEFM) is caused by its use as fluorine-containing building-block for the production of biologically active monofluorinated heterocyclic compounds, for example, 2-fluorocarboxylicacids, 5-fluorobarbituricacidanditsderivatives [1-3]. At present the well- known methods for obtaining 2-diethyl ether of fluoromalonic acid are not without disadvantages such as low values of a yield of the desired product, harsh conditions of synthesis (the use of toxic and corrosive chemicals) [4-9]. As the interest to fluorinated organic compounds is continuously increasing, it is necessary to develop new methods of synthesis, which could remove the mentioned disadvantages. One of the traditional methods of obtaining organofluorine compounds is the nucleophilic substitution of halogen in the corresponding chlorine and bromine derivatives. Metal fluorides are mainly used as a reagent, which allows to exchange chlorine atom by fluorine, generally potassium fluoride [10].
In this article were port results of the research of the reaction of diethyl chloromalonate (DEChM) with potassium fluoride in aprotonic solvents under various conditions.
It is known that the substitution of halogen in the 1, 3 – dicarbonyl compounds proceeds by the mechanism SN1. We carried out theoretical studies of the substitution reaction of chlorine by fluorine as follows:
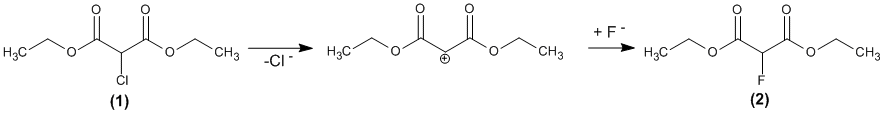
The quantum-chemical calculations of the values of energy barriers in various solvents (aprotonic and protonic) were carried out to estimate the influence of the solvent on the course of the reaction. To take into account the solvation the method SCRF B3LYP/6-311G (d) was used within the model of Tomasi (PCM). The error of this method is ± 3 kJ/mol. The value of a dielectric constant of the solvent is as a criterion which characterizes its.
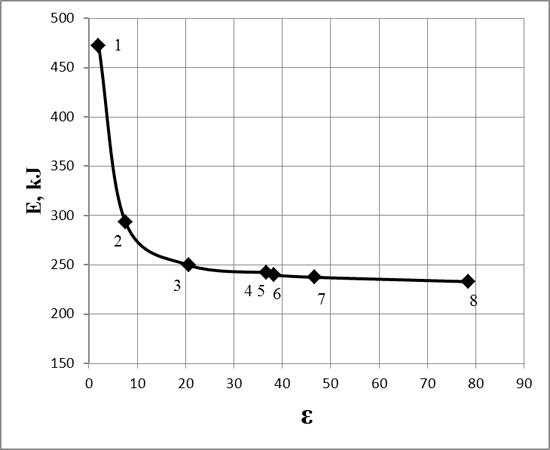
In fig.1 the graph of the dependence of the energy barrier of the reaction on the dielectric constant of the solvent is presented.
 |
|
Fig. 1.The dependence of the energy barrier of the reaction on the dielectric constant of the solvent
As shown in fig.1, the increase of the dielectric constant of the solvent (ε> 20) does not lead to a significant decrease of the energy barrier of the reaction. Thus, polar aprotonic solvents were chosen as effective solvents. They have temperate dielectric constant but do not able to form hydrogen bonds (aceton, acetonitrile, DMF, DMSO).
Based on these data, we carried out a series of experiments in which the reaction conditions (nature of solvent, temperature, reaction time and catalyst) were varied. The composition of the obtained reaction mixtures were analyzed by GC-MS method. The results of these experiments are shown in Figure 1 and Table 1.
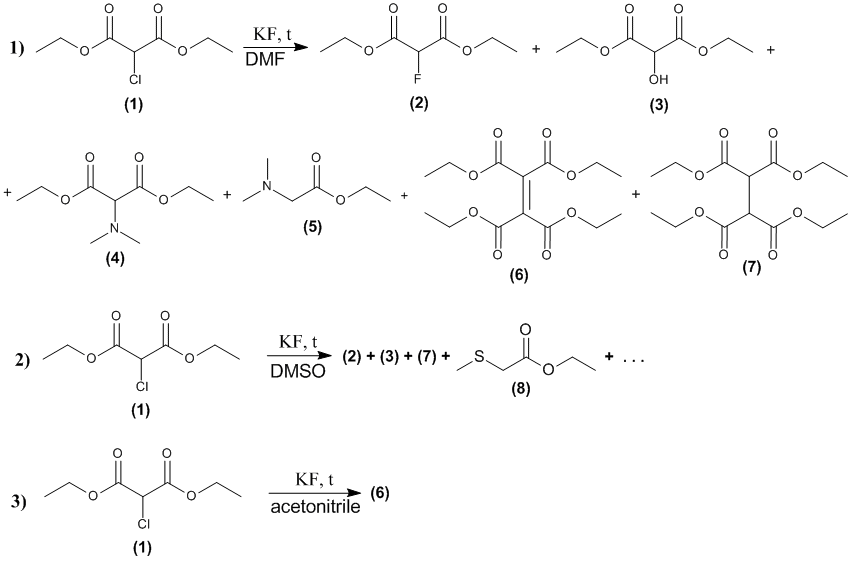
Scheme1.The main reaction products of the reaction of diethyl chloromalonate with potassium fluoride in various aprotonic solvents
The structures of compounds 2, 5, 7, 8 were identified by the database of mass spectra and the structures of the compounds 3, 4, 6 were expected on the basis of an analysis of their mass spectra.
Table 1.
| # | Solvent | Molar ratio DEChM: KF |
Reaction conditions | Concentration of the main reaction products, % | |||||||||
| T, °C | catalyst | t, h | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |||
| 1 | DMF | 2:3 | 150 | — | 6 | 34,8 | 20,6 | 10,8 | 3,4 | —* | 16,7 | — | — |
| 2 | DMF | 2:3 | 100 | — | 24 | — | — | — | 75,2 | 2,6 | — | — | — |
| 3 | DMF | 2:3 | 150 | — | 21 | — | 19,6 | — | — | — | 18,9 | 5,1 | — |
| 4 | DMF | 1:3 | 150 | — | 6 | — | 20,1 | 18,6 | 13,6 | 16,8 | 4,8 | — | — |
| 5 | DMSO | 2:3 | 180 | — | 6 | — | 11,9 | 21,8 | — | — | — | 4,1 | 12,3 |
| 6 | Acetone | 2:3 | 55 | — | 6 | 95,5 | — | — | — | — | — | — | — |
| 7 | DMF | 2:3 | 150 | 18-crown-6 | 6 | 1,5 | 48, 1 | — | — | — | 16,9 | 1,01 | — |
| 8 | Acetonitrile | 2:3 | 80 | 18-crown-6 | 6 | 59,5 | — | — | — | — | 23,3 | — | — |
*—not detected
As follows from the Table 1, the interaction of diethyl chloromalonate with potassium fluoride leads to the formation of various reaction products depending on the reaction conditions. The received results allow us to draw some conclusions about the nature of the influence of these conditions on the studied process.
When carrying out the reaction in DMF, the following processes are observed:
1) the reaction with fluoride anion with the formation of diethyl fluoromalonate2;
2) the substitution reaction of chlorine on the hydroxyl group with the formation of diethyl-2-hydroxymalonate 3;
3) the reaction with the solvent or the product of its decomposition with formation of diethyl-2-(dimetilamino)malonate 4 and the product of its decarboxylation ethyl-2-(dimethylamino) acetate 5;
4) the condensation reactions of DEChM leading to the formation of derivatives of tetracarboxylic acids 6, 7.
When carrying out the reaction at 150°С the content of diethyl fluoromalonate 2 in the reaction mixture is 20 %, while it does not depend on the ration of reagent. With an increase the reaction time from 6 to 21 hours the content of 2 does not change. It was also found that at 150°C, the content of the condensation products 6 and 7 is 20%.
With a decrease the reaction temperature to 100°C the number of side reactions reduced, the reaction mixture mainly contains the compound 4 (75%) and a small amount of compound 5 (2.6%). Diethyl fluoromalonate not detected. It is obvious that at low temperatures DEChM reacts only with the solvent.
However when heating diethyl chloromalonate in DMF (T = 150 ° C) for 6 hours in the absence of potassium fluoride the products of interaction with the solvent or condensation products are not formed. Supposedly KF is involved in these processes.
The reaction in DMSO undergoes with the formation of small amount of diethyl fluoromalonate 2 (12%) and the large number of side products, including ethyl-2-metiltioacetate 8 (12%), formed by the interaction of DEChM with DMSO on the reaction similar reaction Pummerera.
In some cases the compound 3 (20%) is formed. According to the mass spectrum the product 3 was identified as diethyl-2-hydroxymalonate, which is formally the result of hydrolysis. We used pre-dehydrated reagents and solvents, so it is unlikely that the formation of compound 3 due to the presence of water. Perhaps it is the product of secondary processes.
It was established that carrying out the reaction in acetone does not lead to significant changes in the system. Probably the reaction requires higher temperatures.
It should also be noted that in the absence of a solvent diethyl chloromalonate does not react with the KF that is connected with the low solubility of KF in DEChM.
It is known that crown ethers can significantly increase the solubility of ionic compounds in organic solvents. In this case the stable complexes with cations are formed and anions are solvated weakly.
We have shown that the use of 18-crown-6 in the reaction diethyl chloromalonate with KF in DMF (150 °C) leads to an increase in the content of diethyl fluoromalonate by 2.5 times (48%). The solubility of KF in DMF increases in the presence of crown ether due to complexation of the cation of potassium. This leads to increase in the concentration of fluoride anions in solution and to increase the reaction rate of substitution of chlorine by fluorine.
It was found that the reaction in acetonitrile in the presence of 18-crown-6 does not lead to the formation of fluorine-containing derivative 2, and the formation of dimerization product 6 is only observed.
Thus, the influence of the solvent, temperature and the ratio of reagent on the composition of the products was studied. It was shown that the introduction of phase transfer catalyst 18-crown-6 increases the concentration of diethyl-2-fluoromalonate from 20 to 48 %.
The experimental part
The process was carried out in polar aprotonic solvents at a molar ratio of DEChM and potassium fluoride from 2:3 to 1:3, in the temperature range from 55 to 180 °C under constant stirring and heating in a nitrogen atmosphere. Diethyl chloromalonate, 90 %, Alfa Aesar. The analysis of reaction mixtures was performed by gas chromatography-mass spectrometry (GC-MS) on the instrument Trace DSQ (Thermoelectron corp., USA), software Xcalibur 1.4. Conditions of analysis: column BPX5, 25 m, T = 280 °C, and Tthermostat from 40 to 300 °C, V g/c = 1 mL / min, heating rate 10 °/min, scanning 33 - 350 amu.
The general methodology of synthesis
In a three-necked flask of 500 ml equipped with a reflux condenser and mechanical stirrer was charged with 50 g (0.25 mol) diethyl chloromalonate, calculated amount of KF (anhydrous) and 100 mL of solvent. Catalyst 18-crown-6 in an amount of about 1% of the total mass was added. The resulting mixture was heated in a nitrogen atmosphere with vigorous stirring for 6 - 24 hours. After end of the process from the reaction mixture was sampled for GCh-MS.
The research was supported with financial support of the Ministry of Education and Science of Russian Federation within realization Federal Programs «Scientific and scientific- pedagogical shots of innovative Russia» for 2009-2013 years» and «Researches and development in the priority directions of development of a scientific and technological complex of Russia for 2007-2013 years »
References
- Ishikawa N., Takaoka A., Ibrahim K. M. // J. Fluorine Chem. – 1984. – Vol. 25. – Р. 203.
- Devadas B., Kishore N. S., Adams S. P., Gordon J. I. // Bioorg. Med. Chem. Lett. – 1993. – Vol. 3. – P. 779.
- Hutchinson J., Sanford G., Vaughan J. F. S. // Tetrahedron. – 1998. – Vol. 54. – P. 2867.
- Gryszkiewicz-Trochimowski E., Sporzynski A., Wnuk J. // Rec. Trav. Chim. – 1947. – Vol. 66. – P. 424.
- Pattison F.L.M. et al. // Can. J. Chem. – 1965. – Vol. 43. – P. 1700.
- Chambers R. D., Hutchinson J. // J. Fluorine Chem. – 1998. – Vol. 92. – Iss. 1. – P. 45.
- Hiller A., Patt J. T., Steinbach J. // J. Organometallic Chem. – 2006. – Vol. 691. – Iss. 18. – P3737.
- Machleidt H. // Justus LiebigsAnnalen der Chemie. – 1964. – Vol. 676. – P. 66.
- Разработка нового метода синтеза диэтилового эфира 2-фтормалоновой кислоты: Отчет о НИР (промежуточ.) / ТГУ; Руководитель Н. Ф. Мингалимов. ГР 01201177736; Инв. 603063. Томск, 2011. 49 с.
- Hudlicky M., Pavlath A. E. Chemistry of Organic Fluorine Compounds II: A Critical Review. – Washington, DC: Amer. Chem. Soc. – 1995. – 1296 p.
Recommended for publication by S.M. Igoumnov
Fluorine Notes, 2012, 82, 7-8
