Received: May, 2012
Fluorine Notes, 2012, 82, 11-12
Synthesis of Polyfluorine Ethers of Adamantane
G.M. Butov*, S.V. Diakonov*, V.M. Mohov**
*Volzhsky Polytechnical Institute (branch of) VSTU, Russian Federation, 404121, Volgograd region, Volzhsky,
42a Engelsa Street,
e-mail: butov@volpi.ru
**Volgograd State Technical University Russian Federation, 400005, Volgograd, Lenin ave., 28
Abstract: Adamantane containing polyfluorine containing ethers have been obtained by the interaction of 1,3-dehydroadamantane and polyfluorinated alcohols H(CF2)nCH2OH, where n=2,4. The yield achieved was within the range of 68-77%.
Keywords: Polyfluorinated alcohol, 1,3-dehydroadamantane, polyfluorine containing ethers.
Polyfluorinated dialkyl ethers are low-toxic (IV class of danger) and ozone friendly compounds, which can be used as foaming agents, high-temperature heat-carriers, dielectrics, as effective compression, motor, vacuum, lubricating oils and additives [1-4]. The introduction of adamantane radical into the molecule of polyfluorinated ether can significantly improve their exploration characteristics. There is not much information on such ethers [5], researches on both synthesis and properties of such ethers has not been done.
Authors [5] obtained polyfluorine containing ethers of adamantane by the reaction of 1-hydroxiadamantane and polyfluoroalkylchlorosulfites:
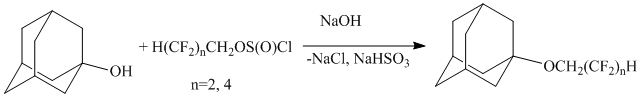
The reaction was carried out in dioxane over sodium hydroxide for 5-6 hours. The yield of reaction was 76%.
The reaction of 1-bromoadamantane and alcoholytes of polyfluorinated alcohols is known [5]:
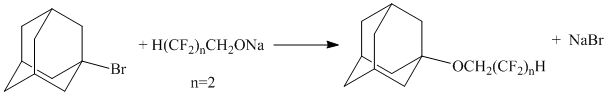
The reaction was carried out in benzene for 5-6 hours. The yield of reaction was 73%.
Those reactions are passing in several stages and are characterized by long duration of 5-6 hours and difficult isolation of products.
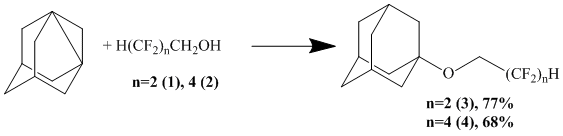
We have developed a convenient and one-stage method to obtain such ethers using reaction of 1,3-dehydroadamantane (DHA) and polyfluorinated alcohols (PA) with common formula H(CF2)nCH2OH, where n=2,4.
2,2,3,3-Tetrafluoropropyl 1 and 2,2,3,3,4,4,5,5-octafluoropentyl 2 alcohols were used as initial reagents.
The synthesis was carried out in the medium of tetrahydrofuran (THF), in the atmosphere of dry, purified from oxygen nitrogen at temperature of 70°С for the period of 30 min at molar ratio equal to 1:2.
In the molecules of polyfluorinated alcohols H(CF2)nCH2OH there are several reaction centres, via which interaction with DHA can take place. These are hydroxyl group, methylene group СН2 (СН-acidic centre), and C-F bonds as well.
Using chromate-mass-spectrometry it has been stated, that the DHA reaction with PA passes preferably by hydroxyl group of the initial alcohol. Apparently, it can be explained by high acidity of polyfluorinated alcohols (for alcohol 1 рka=12,7 [6]), and also by easy protonation of DHA molecule [7].
The DHA interaction products of C-F bond have not been found in the reaction mass. The similar events have been observed earlier in the reaction of DHA with ethyl ether of fluoroacetic acid [8], while ethyl ethers of other halogen containing acids reacted in such conditions [8, 9]. Analogous events have been observed in the reactions with β-dicarbonyl compounds containing trifluoromethyl and difluoromethyl groups [10]. It is interesting, that 1,3-dehydroadamantane interacted along the C-Hal bond with compounds containing atoms of other halogens (Hal = Cl, Вг, I) forming 3-Наl-1-R disubstituted derivatives of adamantane [11-15], and only along C-F- bond reactions did not pass like that. Apparently, this can be explained by small interatomic distance of C-F bond, and also higher energy of bond exceeding the energy of C - H and C – Нal ( Hal = Cl, Br, I) bonds.
The formation of DHA reaction products along the C-H acidic center of polyfluorinated alcohols should have also been supposed, as C-H acidity of CH2-methylene group must be rather high, due to presence of two electron seeking substituents: hydroxyl and polyfluoromethylene ones. It seems like the significantly higher O-H acidity of hydroxyl group and steric difficulties for the attack of methylene group lower the probability of DHA reaction passing by that group. It should be noted, that the interaction of DHA with polyfluorinated alcohols passes without catalyst in short time period (30 min), while the analogous reaction with peroxialcohols [16] of common formula HO-(CH2)n-OO-Bu-t, passes over acidic catalyst in a comparatively longer period of time (180 min), that can be explained by the difference in the acidity of PA as well. As a result of dissociation of hydroxyl group of PA in a solution of THF the forming proton attacks DHA forming adamantly-cation, which easily recombinates with hydroxyl anion of PA forming ether bond.
Thus, DHA selectively reacts with PA by hydroxyl group forming simple ethers: 1-adamantyl-2,2,3,3-tetrafluoropropyl (3, 77%) and 1-adamantyl-2,2,3,3,4,4,5,5-octafluoropentyl (4, 68%), which have been isolated by vacuum distillation. This allows us to speak, that the reaction of DHA with PA is a convinient preparative method to obtain polyfluorine containing ethers of adamantane by joint position allowing obtaining similar compounds in one stage without formation of side and concurrent compounds. The isolation of reaction products is significantly simplified and is driving to the distillation and vacuum distillation of products, that decreases the number of stages and, therefore, the loss of product.
The composition and structure of synthesized compounds are confirmed by NMR 1Н-spectroscopy and chromate-mass-spectrometry. Properties of compounds 3 and 4 corresponded to literature data [5].
The presence of peak of corresponding adamantylcation with m/z 135 with intensity of 37% is a characteristic particularity of mass-spectra of synthesized compounds. At that, most intensive ones are the ions of weight equal to 209 (3) and 309 (4), which correspond to fragment [М-С4Н9]+. This means, that disintegration of adamantane backbone is the main direction for fragmentation of 3 and 4.
The analysis of data from 1Н NMR-spectrum confirms the passing of О-adamantylation, in 1Н NMR-spectrum the signal of proton of hydroxyl group is absent.
Experimental Part
The composition of reaction mass had been studied at chromate-mass-spectrometer «Varian WS».
NMR 1Н-spectrum had been obtained at «Varian» with operational frequency of 300 MHz. DMSO-d6 was used as solvent.
Synthesis of Adamant-1-yl-2,2,3,3-tetrafluoropropyl Ether 3.
To the solution of 3,9 g (0,029 mole) of 2,2,3,3-tetrafluoropropyl alcohol in 20 ml of tetrahydrofuran at room temperature the solution of 2,0 g (0,015 mole) of freshly distilled 1,3-dehydroadamantane in 20ml of tetrahydrofuran was added drop by drop. The solution was kept for 30 min at Т=70°С. The solvent was distilled, the product was isolated by vacuum distillation. We obtained 3,1 g (77%), colorless liquid, b.p. 62°С/1mm, nd15 = 1,4428. Literature data nd20 = 1,4440 [5]. Mass-spectrum, m/z (intensity, %): 266, 35% [M]+; 209, 100% [M–С4Н9]+; 151, 1% [AdO]+, Ad – 1-adamantyl; 135, 37% [Ad]+; NMR 1Н–spectrum, δ, ppm: 1,44¸1,73 m (12H, Ad); 2,11 s (3H, Ad); 3,69 s (2H, CH2CF2); 5,7 s (1H, HCF2).
Synthesis of Adamant-1-yl-2,2,3,3,4,4,5,5-octafluoropentyl Ether 4.
Analogously, to the solution of 7,3 g (0,03 mole) of 2,2,3,3,4,4,5,5-octafluoropentyl alcohol in 20ml of tetrahydrofuran we added the solution of 2,1g (0,016 mole) of freshly distilled 1,3-dehydroadamantane in 20 ml of tetrahydrofuran. The solution was kept for 30 min at Т=70°С. The solvent was distilled, the product was isolated by vacuum distillation. We obtained 3,9 g (68%), colorless liquid, b.p. 81oC/1 mm, nd15 = 1,4434. Literature data nd20 = 1,4442 [5]. Mass-spectrum, m/z (intensity, %): 366, 28% [M]+; 309, 100% [M–С4Н9]+; 151, 2% [AdO]+; 135, 37% [Ad]+.
Physical and chemical properties and parameters of NMR 1Н-spectra of synthesized compounds correlate with the ones listed in the work [2].
References
2. Promyshlennye ftororganicheskie produkty: Spravochnik. B. N. Maksimov, V. G. Barabanov, I. L. Serushkin i dr.L.: Khimiya, 1990.-464s
3. Murata, J.; Yamashita, S. ; Akiyama, M.; Katayama, S. ; Hiaki, T.; Sekiya, A. Vapor Pressures of Hydrofluoroethers. // J. Chem. Eng. Data 2002, 47, 911- 915.
4. Sekiya, A.; Misaki, S. The potential of hydrofluoroethers to replace CFCs, HCFCs and PFCs. J. Fluorine Chem. 2000, 101, 215-221.
5. Sintez 1-poliftoralkoksiproizvodnyh adamantana. Rahimov A.I., Vostrikova O.V. // Zhurnal obshchej khimii. 2011. T. 81. № 2. s. 274-276.
6. Khimicheskaya enciklopediya: V 5 t.: t.4: / Redkol.: Zefirov N.S. i dr..-M.: Bol'shaya Rossijskaya encikl., 1995.-639s.
7. Bagrij E.I. Adamantany. -M. : Nauka, 1989. - 290 s.
8. Vzaimodejstvie 1,3-degidroadamantana s ehfiramiα-galogenkarbonovyh kislot. G.M. Butov, V.M. Mohov, S.V. D'yakonov // Izvestiya VolgGTU: mezhvuz. sb. nauch. st. (Ser. Khimiya i tekhnologiya ehlementorganicheskih monomerov i polimernyh materialov). V. 4, №5/ VolgGTU. – Volgograd, 2007.- s. 30-34.
9. Patent RU 2301796, S07S 67/30, 69/635. G.M. Butov, V.M. Mohov, S.V. D'yakonov. Sposob polucheniya 3–galogen–1– (ehtoksikarbonil)alkiladamantanov. Publ. 27.06.2007 Bull. №18.
10. Issledovanie vzaimodejstviya 1,3-degidroadamantana s alifaticheskimi ftorirovannymiβ-diketonami. Butov G.M., Mohov V.M., Parshin G.Yu., Kunaev R.U. // Izvestiya VolgGTU: mezhvuz. sb. nauch. st. (Ser. Khimiya i tekhnologiya ehlementorganicheskih monomerov i polimernyh materialov). V.4, №5/ VolgGTU.– Volgograd, 2007.-s.25- 27.
11. Sposob polucheniya (3-bromadamant-1-il)alkil- ili arilketonov. G.M. Butov, V.M. Mohov, S.V. D'yakonov, R.U. Kunaev // Izvestiya VolgGTU: mezhvuz. sb. nauch. st. (Ser. Khimiya i tekhnologiya ehlementorganicheskih monomerov i polimernyh materialov). V. 5, №1/ VolgGTU. – Volgograd, 2008.- s. 41-43.
12. Sposob polucheniya Z-chloradamant-1-ilalkilketonov. G.M. Butov, S.V. D'yakonov, G.Yu. Parshin, V.M. Mohov // Izvestiya VolgGTU: mezhvuz. sb. nauch. st. (Ser. Khimiya i tekhnologiya ehlementorganicheskih monomerov i polimernyh materialov). V. 6, № 2. / VolgGTU. - Volgograd, 2009. - s. 40-42.
13. Neobychnoe vzaimodejstvie 1,3-degidroadamantana s 3-brom-1,77- trimetilbiciklo[2.2.1]geptan-2-onom. G.M. Butov, V.M. Mohov, G.Yu. Parshin, R.U. Kunaev // ZhOrKh. - 2007. - № 8. - t. 43. - s. 1256-1257.
14. Vzaimodejstvie 5,7-dimetil-1,3-degidroadamantana s kamforoj i bromkamforoj. Butov G.M., Mohov V.M., Parshin G.Yu., Kunaev R.U.// Izvestiya VolgGTU: mezhvuz. sb. nauch. st. (Ser. Khimiya i tekhnologiya ehlementorganicheskih monomerov i polimernyh materialov). V.4, №5/ VolgGTU.– Volgograd, 2007. - s. 22-24.
15. O vzaimodejstvii chlorangidridov aromaticheskih karbonovyh kislot s 1,3-degidroadamantanom. G.M. Butov, V.M. Mohov, S.V. D'yakonov // Izvestiya VolgGTU: mezhvuz. sb. nauch. st. (Ser. Himiya i tekhnologiya ehlementorganicheskih monomerov i polimernyh materialov). Vyp. 8, № 2 / VolgGTU. - Volgograd, 2011. - s. 27-28.
16. Sintez prostyh 1-adamantilovyh ehfirov peroksispirtov B.I. No, G.M. Butov, S.M. Ledenev. // ZhOrKh. - 1992. - № 3. - t. 28. - s. 606-607.
Recommended for publication by Prof. Alexander I. Rakhimov
Fluorine Notes, 2012, 82, 11-12
