Received: March, 2012
Fluorine Notes, 2012, 82, 3-4
FLUORINATION OF NANOSIZED AMORPHOUS SILICA DERIVED FROM SILICATE RAW MATERIALS
L.P. Demyanova, *A. Tressaud, *Labrugere C., *Durand E., **Majimel J., Petsyk P.A., Kozlov K.G
Institute of Geology and Nature Management FED-RAS, 1 Relochny, Blagoveschensk 675000, Russia
e-mail: larisa-demyanova@ascnet.ru
* ICMCB-CNRS, University Bordeaux, 87, Avenue Dr. Albert Schweitzer, Pessac, 33608 France
** CREMEM, Microscopy Center, University Bordeaux, France
Abstract: Surface properties of nanosized silica, synthesized using fluoridation technologies, have been modified by direct fluorination using F2鉾as or by plasma radio frequency. The modifications of the physicochemical characteristics of the surface have been characterized by spectrometry of photoelectrons (XPS), infrared and high resolution transmission electronic microscopy and compared with those of pristine silica.
Keywords: silicates, amorphous silica, fluorination
INTRODUCTION
In connection with the development of nanotechnologies related to silica and quartz-based raw materials, a special interest has been raised concerning their surface modification. Properties of various types of amorphous silica with high added mostly depend on the reactions occurring at the surface. These materials are very important in the technology of catalysts cracking, processing of raw minerals, use in potteries and adsorbents. Amorphous silica is also directly utilized in the manufacture of siliceous fillers and thickeners in organic systems, including paints, inks, elastomers and lubricants. The modulation of the hydrophobic-hydrophilic surface properties allows bringing a great value to the silica materials. Also, by acting on the adsorption processes onto the surface, either positive or negative superficial charges can promote valuable improvements of silica properties. Waterproof and reflecting properties of SiO2 have been also investigated. Within these last 50 years, a clear picture of the nature of the silica surface has been thus obtained, and on the basis of the chemical modification of this surface, new products could be proposed [1, 2].
It is known that C–Fn groups increase waterproof character of materials [3]. Recently, fluorination processes have been successfully applied to mesoporous silica [4, 5] and alumino-silicate minerals [6, 7] using various fluorinating routes.
The starting materials concerned by the present work, are amorphous silicas, prepared using fluoridation technologies from quartz or kaolin raw minerals of Amur region (Russia). The purpose of this work is to study the changes occurring on the surface of amorphous silica, by fluorination treatments using either direct F2-gas, or c–C4F8 radio frequency (rf)-plasmas.
Changes of the superficial properties of amorphous silica have been studied by means of several physical methods: X-ray photoelectron spectroscopy (XPS), which allows to study changes of the binding energies of elements present on the surface, infra-red spectroscopy and electron microscopy.
EXPERIMENTAL PROCEDURE
Amorphous silica has been obtained from silica-based raw materials of the Amur region (Russia), namely quartz sand and kaolin. The purification and transformation of quartz sand and kaolin into pure SiO2 has been developed at Institute of Geology and Wildlife Management (IGeM - RAS) by a fluoridation method using NH4HF2 [8, 9, 10 and others]. The chemical composition of the two types of amorphous silica [11] is presented in Table 1. Their specific surface is 98 m2/g, with a particle size of 20 nm and a level of impurity less than 1∙10-4 weight % [12].
Table 1. Chemical analysis of amorphous silica, obtained by fluoridation technology either from quartz sand or from kaolin
Amorphous silica |
|||
Ex-quartz |
Ex-kaolin |
||
| Si Al Fe Li Na K Ti Mn Mg Ca P |
41, 86 % 700 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm |
Si Al Fe Li Na K Ti Mn Mg Ca P |
40, 85 % 0.25 % ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm ≤ 500 ppm |
Samples of amorphous silica have been further treated by fluorination using either F2-gas or c砲4F8 in radio frequency (rf) plasma conditions. These experiments were carried out at ICMCB-CNRS (Bordeaux) on equipments designed and developed there.
Direct F2-gas fluorination
Direct F2-gas fluorination process was performed at 100°C in a "fluorine line" using handling procedures previously described, with adapted experimental set-up enabling to work in excellent safety conditions [13]. The samples were set in a Ni boat which had been previously passivated. F2-gas, 50% diluted in N2 (Air Products) was used at room pressure. The reaction duration depended on the starting materials but generally was of 60 min, in order to limit the fluorination to the surface only. At the end of the experiment, F2-gas was eliminated from the reactor and replaced by N2.
Cold rf-plasma fluorination
Rf-plasma fluorination is a low-temperature process where fluorinated gases are excited by an rf source and dissociated into chemically active atoms, radicals and molecules [14]. Among the possible fluorinated gases, c-C4F8 (octafluorocyclobutane) was selected and excited by an rf source at 13.56 MHz. A primary vacuum was obtained by a 40 m3/h pump equipped with a liquid nitrogen condenser, which trapped the residual gases. The reactor comprised two cylindrical barrel-type aluminium electrodes which were coated with alumina and which were located within a distance of 2 cm from each other and several gas inlets allowing the use of gas mixtures. The inner electrode was connected to the rf source, the outer electrode was grounded. The sample to be treated was placed at the center of the chamber, onto the inner electrode. The gas was introduced in the inner part of the reactor and then dissociated by electron impacts occurring between the two electrodes. Neutral species and radicals diffused from this plasma zone to the center of the reactor where they reacted with the sample. The samples are generally pre-treated with O2 plasma before plasma fluorination process, in order to render their surface more reactive. This pre-treatment removed adsorbed airborne organic pollution and filled oxygen vacancies at the surface of the sample.
Under rf plasma conditions, c-C4F8 molecules dissociate into neutral radicals (e.g., CF, CF2, CF3), ions (C2F4+), and stable molecules (e.g., C2F, some non-dissociated C4F8). It has been shown using in-situ spectroscopic diagnosis that CF2 species are the dominant CFx radicals [15], which further deposit onto the treated material, forming a fluorinated layer of average CF2 composition.
Recent studies dealing with octafluorocyclobutane rf plasmas have confirmed that the film growth is controlled by CxFy ions and neutrals. The efficiency of the film deposition was investigated in terms of size of the monomer precursors, in particular C2F4+ ion species [15, 16]. In the case of SiO2 substrates, the adhesion of the film can be drastically improved by inserting an adhesion promoter consisting of Si-rich SiO2 [3]. The adhesion performances can be generally evaluated by peeling experiments. Unfortunately such a characterization could not be performed in our case because of the small size of particles.
Parameters that may be varied during the plasma fluorination process are pressure and flow of the gases, temperature and reaction duration [17]. All plasma processes were carried out with an rf power of 80 W. The pressure of the gas in the reactor was around 100 mTorr, the temperature was maintained at room temperature, whereas the duration of the treatment was one hour [18]. It should be noted that in most cases, an important volatilization of the starting product was observed, due to the formation of SiF4.
Morphological, physical and chemical characterizations
The morphological, physical and chemical changes occurring on the surface of pristine silica and fluorinated samples, have been investigated by means of the analysis of X-ray photoelectron spectroscopy (XPS), giving information on the shift of binding energies (BE) of the various elements present onto the surface (accessible depth of a material ~ 100 nm). XPS analyses were performed with a VG 220 i-XL ESCALAB. The radiation was an Mg non-monochromatized source (1253.6 eV) at 200 W. 200-300 mm diameter areas were investigated on each sample. The insulating character of the silicate samples needed low-energy (4-6 eV) electron compensation. Survey and high resolution spectra were recorded with pass energy of 150 and 20 eV, respectively. When fitting the XPS envelopes, each component was considered as having similar FWHM, i.e. 1.5 eV. This procedure appeared to be the most reliable one, as previously proposed in investigations on fluorinated carbon materials [19]. A good agreement between the experimental curve and the full calculated envelope was obtained, which allowed explaining in addition subtle distinctions between the proportions of fluorinated carbon components. As these materials are non-conductive, flood gun was activated onto the samples to shift the high-resolution spectra in their normal range (i.e. 285–293 eV for C1s).
The quantitative analysis of basic elements Si, O, F, C, was determined by means of "Advantage processing software" of Thermo Fisher Scientific. The BE values were generally determined with respect to the Si 2p BE in silica and silicates: BE = 103.5eV.
Characteristics of adsorption of fluorinated amorphous silica, in particular the change of superficial hydroxyl groups, were defined by means of IR-spectroscopy with use "Spectrum One" IR-spectrometer, Perkin-Elmer Corp. 2002. Recording were measured in the 4000 – 400 cm-1 range, with 4 cm-1 steps and 16 scans min-1.
The morphological analysis of the materials was carried out with High Resolution Transmission Electron Microscope (HRTEM) images on scanning microscope JEOL 2200 FS( 200 KV), with Field Emission Gun (FEG) mode .
RESULTS AND DISCUSSION
The species present on the surface of silica samples are of great importance because they form a zone of direct interaction with the environment and/or other materials. Oxidation, corrosion, wear, activation and other processes begin on the surface, and concerning certain properties only the surface is concerned, for example in the coupling with the surface of other materials. Even a small amount of surface pollution can change considerably the behavior of the material and depending on the type of adsorption processes that lead to either positive or negative superficial charges, the properties may be drastically changed.
However it is very difficult to deposit a thick layer on any kind of silica, because of instant evaporation of gaseous silicon tetrafluoride (SiF4). In both cases it has been noted that the fluorine amount fixed on the surface reached very high levels, up to 13 %.
After processing by c–C4F8 rf-plasma, the sizes of silica particles keep their nanometric character. By HRTEM it is shown that the average size of amorphous silica particles is maintained around 20 nm (Fig. 1). A small dispersion of the sizes however can be noted. This effect can be connected with the two possible reactions: CF2 deposition vs. reactive etching, that lead to the formation of globular particles with various dimensions.
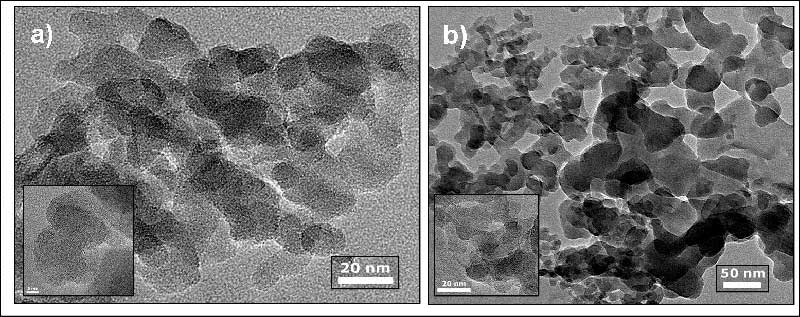
Fig. 1. HRTEM images of pristine amorphous silica obtained from fluoridation (NH4HF2) technology a), and after treatment by c–C4F8 rf-plasma b).
The elemental analysis at the nanometric level also has been carried out by EDX using the same equipment (Fig. 2). In the left part of the figure, the electronic images of the various elements: Si, F, O are grouped. It can be noted that fluorine is homogeneously distributed in all particles. In the right part, the power dispersive spectrum of the various components is presented with 11.6 atom.% fluorine (ex-quartz sand SiO2).
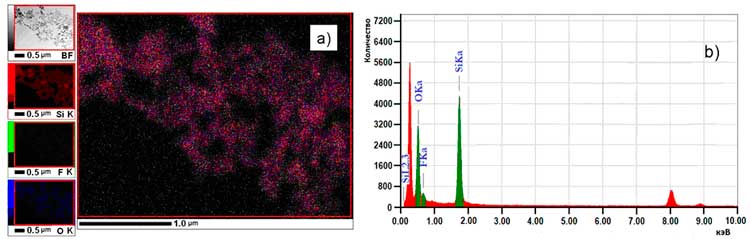
Fig. 2. Elemental analysis at the nanometric level of amorphous silica (ex-quartz sand SiO2) processed by c–C4F8 rf-plasma a) and power dispersive spectrum b).
Using both routes, that is F2-gas or c–C4F8 rf-plasma, similar quantity of fluorine are found onto the surface of silica particles: about 12–13 atom.%. This amount is the most important ever observed at surface of fluorinated nano-silicas. XPS spectra corresponding to Si2p, O1s, F1s and C1s have been recorded and fitted (Fig. 3). On spectrum C1s some pollution carbon can be found. It is connected with presence of C–Fn bonds which interferes with the further fastening of carbon or hydroxyl groups. In case of processing with rf-plasma, at ~ 290 eV there is a noticeable presence of covalent C–Fn bonding, as found in carbon fluorides or fluoro-polymers. This result is related to the deposition of a fluorocarbon layer on the surface of amorphous silica particles. These species are predictably formed by the cracking of the c-C4F8 cycling molecule in rf plasma conditions. The surface elemental analysis (Si, O, F and C) of amorphous silica as-received or F-treated, deduced from XPS results are presented in Table 2.
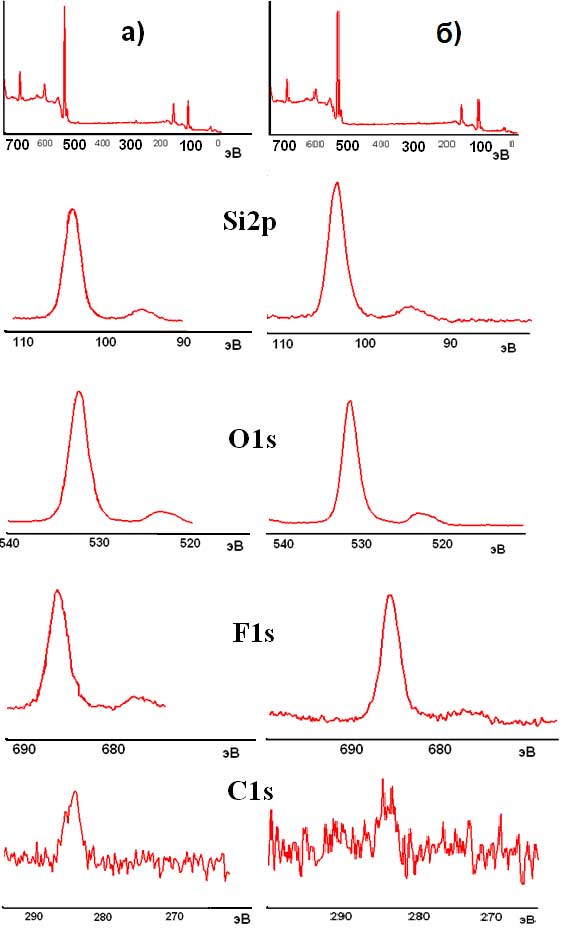
Fig. 3. XPS surface spectra nanometric silica amorphous the fluorinated: F2-gas (a) or c砲4F8 high frequency-plasma (b)
Table 2. Surface elemental analysis of amorphous silica (average error of XPS analysis ± 0,05 atom.%)
Sample |
Elemental analysis, atom.% |
F/Si |
|||
Si |
O |
F |
C |
||
Ex-quartz amorphous silica (АКQ) |
30.6 |
60.1 |
7.6 |
1.6 |
0.25 |
АКQ treated F2–gas, 1 h., 100 °C |
29.6 |
54.9 |
13.6 |
1.9 |
0.46 |
АКQ treated c–C4F8 rf-plasma |
30.1 |
55.6 |
11.6 |
2.8 |
0.38 |
Ex-kaolin amorphous silica (АКK) |
30.1 |
59.4 |
8.5 |
1.9 |
0.28 |
АКK treated F2–gas, 1h., 100°C |
27.5 |
56.4 |
14.1 |
2 |
0.51 |
АКK treated c–C4F8 rf-plasma |
29.3 |
54.5 |
14.3 |
1.9 |
0.49 |
IR-spectra are compared in Fig. 4. The modification of the bands accounts for structural transformations, and changes of properties of the amorphous silica once fluorine treated.
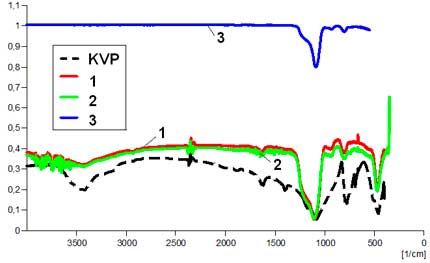 |
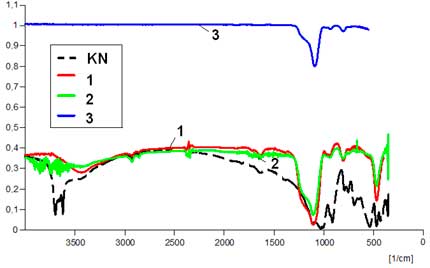 |
Fig. 4. IR spectra: initial raw materials (KVP-quartz sand, a KN-kaolin); starting amorphous silica obtained from the fluoridation (NH4HF2) technology [1]; amorphous silica samples after direct F2-gas fluorination (green curves) [2], or after c-C4F8 rf-plasma fluorination (blue curves) [3], for ex-quartz (a) or ex-kaolin silica (b).
Absorption bands of С-Н valence fluctuations, in the 2800 – 3000 cm-1 range are due to pollution occurring during the preparation of samples for the analysis. Information in the 400 to 1600 cm-1 range characterizes structures of nuclear groups present in the investigated products. The investigated series of samples are in good agreement with the spectral picture of β-quartz: that is an intensive band in the 1167–1080 cm-1 range, a doublet around 803-780 cm-1, a weak line at 695 cm-1 and a rather intense band about 465 cm-1. In both initial samples of amorphous silica obtained from quartz and kaolin minerals and in fluorinated samples of amorphous silica, the absorption band ν = 850–1100 cm-1 which corresponds to valence fluctuations of SiO4 tetrahedra [20] can be noted.
The absorption band at ν = 730–760 cm-1 corresponds to valence fluctuations of SiO2 groups, and the band at ν = 500 cm-1 and lower frquencies, to deformation fluctuations of this group. The fluorinated samples have absorption bands at ν = 478 cm-1 and ν = 710 cm-1 corresponding to bond fluctuations of [SiF6] groups.
In every case, except for sample fluoridated with c–C4F8 in rf-plasma conditions, IR spectra contain some bands related to the presence of adsorbed water. On the spectra, these bands occur at (ν =3240–3500 cm-1) for the valence fluctuations of adsorbed H2O molecules, and deformation fluctuations of adsorbed H2O occur at (ν =1600–1640 cm-1) [21]. These spectra show distinctive properties between initial amorphous silica, obtained from quartz or kaolin and with those whose surface has been modified by F2-gas. Absorption bands at ν =478 cm-1 , ν =710 cm-1 and ν =930 cm-1 have been identified and correspond to Si–F bonds of groups [SiO6–XFX] [7]. These spectra show some clear differences between initial and surface-modified samples. They can be considered as related to change of the adsorbed water and hydroxyl groups. In the 3000–3500 cm-1 range, the treated samples have a more flat baseline of the spectrum, and also the line looks like a broken curve that accounts for the presence of a protective film on a surface of amorphous silica. From a physical and chemical viewpoint, it becomes obvious that the waterproof properties after fluorination treatment have to be considered.
CONCLUSIONS
On the basis of the results on the influence of the fluorination treatment on amorphous silica, it is possible to draw the following conclusions. Direct fluorination by F2-gas allows bringing a significant amount of fluorine on the surface of amorphous silica, about 13 atom.%. Si–F bonds are present on superficial groups within [SiO6–XFX] and substitute Si–OH bonds. In addition, amorphous silica obtained from kaolin is more stable vs. surface modification by F2-gas. It can be noted that the post-fluorination treatments do not modify the size of silica particles, whose average value is around 20 nm. The fluorinated amorphous silica has waterproof properties, in comparison with initial untreated samples, prepared from quartz or kaolin using fluorination technology. The samples fluorinated by F2-gas silica amorphous have waterproof properties, in comparison with initial samples of amorphous silica. In the of case fluorination with octafluorocyclobutane in rf-plasma conditions, IR-spectra of samples show the absence of absorption bands of valence and deformation of adsorbed H2O molecules. Finally, the interest to create waterproof layers onto nanopowders of amorphous silica is one of the most important features: in addition the final fluorinated products may be thermally stable up to 300–400°C, as shown earlier [5].
LITERATURE
- Zhang Ye, Chandra Kambhamettu: 3D head tracking under partial occlusion // Pattern Recognition 35 (7) (2002) 1545-1557.
- Zhang Ye, Chandra Kambhamettu: Stereo Matching with Segmentation-Based Cooperation // ECCV (2) (2002) 556-571.
- Tatsumi T., Endo K. // J. Photopolym. Sci; Technol., 12 (1999) 193-198.
- Lataste E., Demourgues A., Leclerc H. et al. // J. Phys. Chem. (2008) C 112. P. 10943.
- Lataste E., Legein C., Body M. et al. // J. Phys. (2009) C 113. P. 18652.
- Demyanova L. P., Tressaud A. // J. Fluor. Chem. (2009) 130. P. 799.
- Tressaud A., Labrugère C., Durand E. et al.// J.Vac. Sci.Technol. (2010) A 28, P. 373.
- Melnichenko E.I. Ftoridnaja processing rare-metal ores of the Far East / Vladivostok, Publishing house: Dalnauka. (2002) 267 p.
- Buinovskii A.S., D'Yachenko A.N., Pogrebenkov V.M. >Fluorid technology for fabrication of mullite articles from quartz-topaz //Glass and Ceramics. Vol. 63. N 11-12. (2006) 419-421.
- Rimkevich V.S., Malovitskiy Yu.N., Demyanova L.P. // Patent RF № 2286947, N 31. (2006) 34 p.
- Demyanova L.P., Makeeva T.B., Buynovsky A.S. Structural and phase changes natural quartz crystal in the fluoride treatment // Steclo i ceramic. № 9. 2011. P. 32-35.
- Demyanova L.P., Tressaud A., Buzare J.-Y. et al. // Inorganic Materials. Vol. 45. N 2. (2009) 151-156.
- Grannec J., Lozano L., Chapter 2 // "Inorganic Solid Fluorides", P. Hagenmuller Ed., Academic Press. (1985).
- Plumb I.C., Ryan K.R. // Plasma Chem. Plasma Process. 6, (1986) 205.
- Tajima S., Komvopoulos K. // J. Phys. Chem. C 111 (2007) 4358-4367.
- Kono A., Ohya Y. // Jpn. J. Appl. Phys. 39 (2000) 1365-1368.
- Cardinaud C., Tressaud A. // Chapter 14, "Advanced Inorganic Fluorides", T. Nakajima, B. Zemva, Tressaud A. // Eds. p 437, Elsevier (2000).
- Tressaud A., Labrugère C., Durand E. et al. // Science China, Ser. E-Technolog. Sciences, 52, (2009) 104-110.
- Nansé G, Papirer E, Fioux P et al. Fluorination of carbon blacks, Carbon 35 // Amsterdam: Pergamon-Elsevier, (1997) 175-194, 371-388 and 515-528.
- Little L. H. Infrared Spectra of Adsorbed Species / Academic Press. (1972) 460.
- Nakamoto K. Infrared Spectra of Inorganic and Coordination Compounds / Wiley. (1991) 535.
Recommended for publication by Member of the of Russian Academy of Sciences Prof. Viacheslav M. Buznik
Fluorine Notes, 2012, 82, 3-4
