Received: March, 2012
Fluorine Notes, 2012, 81, 7-8
THE ETHERIFICATION OF THE ALCOHOL-TELOMERS (n= 3 and 4) WITH ABIETIC ACID
L.M.Popova1, S.V.Vershilov2
1Saint-Petersburg State Technological University of Plant Polymers, Saint-Petersburg, Russia
2State Enterprise S.V.Lebedev Research Institute of Synthetic Rubber (NIISK), Saint-Petersburg,
Russia
e-mail: lorapopova@mail.ru
Abstract: Polyfluoroalkyl-containing esters abietic and dehydroabietic acids were synthesized by the reaction of 1H,1H,7H-perfluoroheptan-1-ole and 1H,1H,9H-perfluorononan-1-ole and abietic acid without solvent in acid catalysis. The structure of the esters was confirmed by IR, H1 and F19-NMR spectroscopy.
Keywords: Abietic acid, polyfluoroalkyl-containing esters abietic and dehydroabietic acids.
A permanent increase of hydrocarbons value gave an additionnal impulse to the research on complex remaking of vegetable raw materials. Crude rosin acids, being rich in tricyclic carbon acids, are a suitable source of many organic chemicals.
It is well-known, that properties of rosins (colophonys) esters depend on a kind of the rosin and a structure of the alcohols used. Different kinds of esters are used in production of synthetic resins, plastificizers, varnishers, paints etc. They are ecological products [1, 2].
Introduction of long-chain fluorinated fragments into an organic substance confer to it surface activity [3]. For this reason the fluorine contained colophony esters with such fluorinated alcohols may be oleo- and/or hydrofobizing agents, for example, of fibrous composites.
Previously [4], 1H,1H,5H-trihydrooctafluoropentyldehydroabietate was produced by esterification of 1H,1H,5H-trihydrooctafluoropentan-1-ole with dehydroabietic acid in presence of H2SO4 conc. More over, 2,2,2-trifluoroethyl-, 1H,1H,3H-trihydrotetrafluoropropyl-, 1H,1H,5H-trihydrooctafluoropentylabietates and – dehydroabietates and perfluorophenylabietate and dehydroabietate were synthesised by interaction of abietic acyl chloride with corresponding fluorocontained alcohols in form of sodium salts [5].
Similarly tall rosin esterification [6], polyfluoroalkyl contained esters (II and III) were synthesized by interaction of 1H,1H,7H-trihydrododecafluoroheptan-1-ole and 1H,1H,9H-trihydrohexadecafluorononan-1-ole with abietic acid (I), scheme:
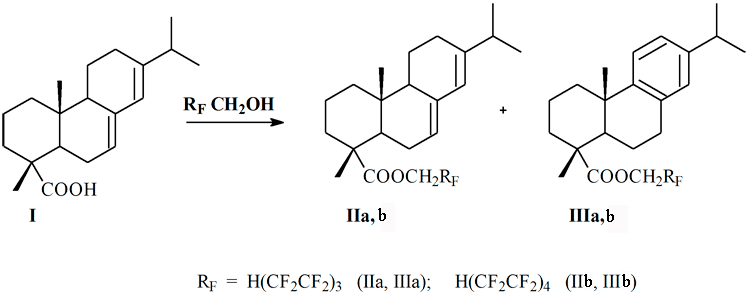
The esterification of equimolecular amounts of the reagents carried out in presence of H2SO4 conc. as catalyzer with no solvents. After heating at 170-200°C during 5-6 h., the reaction mass was cooled, dissolved in 50 ml of diethyl ether, washed containeously with 5% aqueous sodium hydrocarbonate solution and distilled water to neutral pH value. Then ether solution was dried with Na2SO4, filtered and evaporated. The TLC method was used to control the reaction. In all cases glassly resins were formed, total issues of II and III riched 90%.
The structures of synthesised compounds were estimated by means of IR and NMR 1H and 19 F methods.
The absorption peaks in the region 2997-2322 sm-1 assigned to the νC-H bonds. Skeleton vibrations of aromatic ring (νC=C and δCH) appear in the region 1488-1385 cm-1, and strong absorption found in the region 1749-1695 cm-1. Absorption associated with C-F stretching frequencies is found in the region of 1200-1134 cm-1. This a normal region for such absorption to occur (see table).
Table. Analytical and spectra data of abietic acid (I) and their esters (II and III).
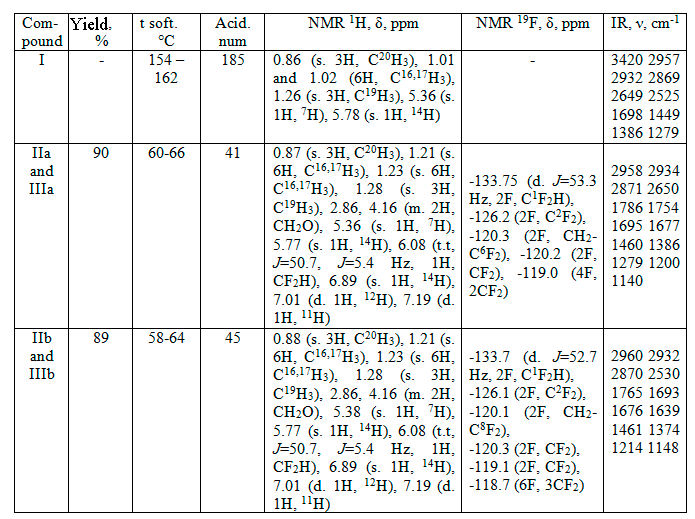
The 1H NMR spectra of the esters (II and III) showed characteristic multiplete absorption peaks at 4,16 ppm instead of usually observed triplete, that is probably connected with diastereomeric structures formation of the –OCH2- group in polyfluoroalkyl fragment. An olefin bonds conjugated protons signals appear at 5.36 (IIb) and 5.38 (IIa) (C7H), and 5.78 (C14H) (II) ppm.
One singlet absorption peak at 6.89 ppm (C14H) and two doublet peaks at 7.01 (C12H) and 7.19 (C11H) ppm may be assigned to aromatic ring in the structure of (III).
Moreover, triplet absorption peak with chemical shift 6.08 ppm is assigned to the protons of terminal –CF2H group (see table).
Although the starting abietic acid (I) contains about 10% of dehydroabietic acid (spectral data), the main products of the esterification process were dehydroabietic acids derivatives (III) (about 90%). Analogous behavior, i.e. acid promoted didisproportionation of abietic acid under heating, was observed formerly [6, 7],
The 19F NMR spectra of (II and III) shows a doublets with chemical shifts –133.75 ppm (JHF 53.3 Hz) (IIa and IIIa), and –133.7 (JHF 52.7 Hz) (IIb and IIIb), assigned to -CF2H groups resonance signal of fluorine atoms. The signals of fluorine atom in methylene group C2F2 appear as singlets at –126.2 ppm (IIa and IIIa), and –126.1 ppm (IIb and IIIb). The signals of other -CF2- groups fluorine atoms appears in the region from –120.3÷–118.7 ppm. Theirs multiplicity and integral intensity corresponds to polyfluoro alkyl telomeric fragments (see table).
Experimental part
TLC method was used to control for the reaction: Sorbfil, hexane : methilene chloride : acetone (1 : 1 : 0,5). IR spectra were registered on Shimadzu IR Prestige-2 (solutions in CCl4). 1H and 19F NMR spectra were recorded on Bruker 500 at frequency 500 MHz (19F NMR at 470 MHz), in CDCl3, external standart – CCl3F. Commercial 1H,1H,7H-perfluoroheptan-1-ol (a) and 1H,1H,9H-perfluorononan-1-ol (b), so-called telomers (n=3 and 4, corresp.) were used as starting materials.
1H,1H,7H-Dodecafluoroheptylabietate (IIa) and -dehydroabietate (IIIa). To 5.0 g (17 mmol) of abietic acid (I) was added 6,0 (18 mmol) of 1H,1H,7H-dodecafluoroheptan-1-ole (IIa) and 2-3 drops of. H2SO4 conc. under argon atmosphere. After stirring for 5 h at 170°C, the reaction mass was extracted with diethyl ether (3×20 ml) at room temperature. Solution obtained was washed with aqueous 5% sodium hydrocarbonate, then with water to neutral reaction and dryed under Na2SO4. Then the solvent was evaporated. The residue was maintained in vacuum under P2O5, to give 9.0 g (90% exit) of pale-yellow amorphous solid, softened at 60-66°C. Analytical and spectral data presents in table.
1H,1H,9H-Hexalecafluorononylabietate (IIb) and – dehydroabietate (IIIb). These esters were prepared in the same manner as (IIIa): from 7,8 g (18 mmol) of 1H,1H,9H-hexadecafluorononane-1-ole (II during 6 h at 200°C. Yield 10.5 g (89%), light-yellow amorphous solid softened at 58-64°C. Analytical and spectral data presents in table.
Conclusion
The interaction between fluorine contained alcohols and abietic acid in acidic media at 170-200OC led to forming of corresponding dehydroabietic esters in good yields (89-90%).
References
- 1. Vladimirova T.M., Tretyakov C.I., Zhabin V.I., Koptelov A.E. Preparation and processing of thall olls materials: monograpf Arkhangelsk: Arkhangelsk State Teknical University Pubishing, 2008. 155 p.
- A.M. Al-Sabagh, T.T. Khidr, A.M. Atta // Petroleum Science and Technology. 2002. V. 20. N 7-8. P. 693-711.
- Ishikawa N., Kobayashi Y. Fluorine compounds. Chemistry and application. M.: Mir, 1982. 276 p.
- L.M. Popova, I.N. Gaydukov // Russian Journal of Applied Chemistry (Zhurnal Prikladnoi Khimii), 2008. V. 81, Iss. 2. P. 338–339.
- I.N. Gaydukov, I.N. Gaydukov // Russian Journal of Applied Chemistry (Zhurnal Prikladnoi Khimii), 2008. V. 81, Iss. 12. P. 2065–2067.
- L.M. Popova, U.A. Dmitrieva, C.V. Vershilov //Russian Journal of Applied Chemistry (Zhurnal Prikladnoi Khimii), 2011. V. 84. Iss. 2. P. 329-332.
- Kryachko E.N., Trishin U.G., Popova L.M. / Book of Abstracts V Rus. Research Conf. "Chemistry and technology of plant compound". Ufa, 8-12 June 2008. P. 171.
Recommended for publication by Vadim Kornilov
Fluorine Notes, 2012, 81, 7-8
