Received: January, 2012
Fluorine Notes, 2012, 81, 5-6
About the mechanism of electrochemical synthesis of perfluoroalkylbromide
O.N. Chechina *, V.V. Berenblit **, S.V. Sokolov ***
* Samara State Technical University,
** Federal State Unitary Enterprise NIISK after S.V.Lebedev,
*** CJSC "Anles"
e-mail: chechinao@yandex.ru
Abstract:Measurements of the electrode polarization and rhe preparative electrolysis evidenced that perfluoroalkyl bromide can be obtained by the electrolysis of perfluorocarboxylic acids in water-acetonitrile medium containing sodium bromide on the platinum anode due to the electrochemical desorption of the absorbed bromine atoms during the discharge of the perfluoroalkyl carboxylic anion.
Keywords: perfluoroalkylbromide, perfluorocarboxylic acids, synthesis Kolbe, bromine, acetonitrile, radiopaque substance, flame arresters, polarizing researches, preparative electrosynthesis, electrochemical desorption, platinum-anode.
Perfluorocarbons containing bromine atoms are effective flame arresters. They have been used to protect personnel and valuable electronic equipment if the ignition occurs in a confined space [1]. Because of the lability of the bromine atom both in radical and ionic reactions it is possible to use its perfluoroalkyl compounds in the synthesis of new organofluorine compounds. Perftoroktilbromid has been used as a radiopaque component in gas transfer liquids in medicine [2].
A number of chemical methods have been represented for producing substances that require stringent conditions and the use of complex equipment. These substances do not provide sufficient purity and reproducibility of the title product properties [3].
The Kolbe electrosynthesis has been successfully used for the synthesis of different perfluorochemicals [4-6]. This method does not require high temperatures and pressures, and provides good reproducibility under mild conditions of electrolysis.
It is known that elektrochemical version of Hunsdiecker-Borodin reaction with the formation of corresponding perfluoro alkyl halogenide is carried out by the electrolysis of solutions of partially neutralized perfluorocarboxylic acids and halogens in the medium of a mixture of acetonitrile-water (1:1) [7]. The use of perfluorononan acid in the presence of elemental bromine on a platinum anode at a current density of 0.01-0.03 A/cm2 allowed to obtain perfluoroalkyl bromide in 94% yield. [8].
In the presence of elemental chlorine a wave of halogen oxidation appears in the polarization curve on platinum. The wave is at least 1 volt previous to the adsorption and discharge of perfluoroalkyl carboxylic anion. It has been suggested that the adsorption of halogen and the discharge of anions fluorocarbon acids occur at different parts of the anode surface with different power characteristics.
Anode condensation of perfluoroalkyl radical with the bromine radical on the platinum anode apparently occurs according to the mechanism of electrochemical desorption:
Br2 (dissolv.) + Pt → (Pt)Br· ads (1)
(Pt)Br· ads + RFCOO- → RFBr + CO2 + e- (2)
Sufficiently low rate of the process is limited by the low concentration of bromine free radicals. The process is accompanied by a substantial yeild of Kolbe dimer due to the adsorption of perfluoroalkyl carboxylic radicals in the lack of bromine radicals:
RFCOO·+Pt → (Pt)RFCOO·ads+e· (3)
(Pt)RFCOO·ads +RFCOO·→ RF-RF + 2CO2 +e· (4)
So we tried to intensify the electrosynthesis of perfluorooctyl bromide under conditions of anodic oxidation of perfluorocarboxylic acids by increasing the concentration of adsorbed bromine radicals on the anode surface under conditions of the combined discharge of the anion of perfluorocarboxylic acids and inorganic bromide ion. Adsorption of bromine radical is not an obstacle to the discharge of the anion of fluorocarboxylic acid. Under conditions of excess of adsorbed bromine radicals on the anode the optimum conditions are realized for their electrochemical desorption under conditions of the anion discharge of perfluorocarboxylic acids with condensation of bromine with fluoroalkyl radicals:
Br- → Br·ads+ e· (5)
Br·ads+ RfCOO· → RfBr + CO2 + e· (6)
Electrochemical desorption of bromine with more or less yield due to the dimerization of its radicals would likely occur simultaneously with the reaction:
Br·ads+ Br- → Br2 + e· (7)
EXPERIMENTAL
The mixture of acetonitrile - (CH3CN; TU 6-09-3534-87) purified due to the method of [9] and distilled water in the concentration range from 90 to 30% by volume has been used as the electrolytic medium for polarization measurements and preparative electrosynthesis.
Perfluorooctanoic (pelargonic) acid (CF3(CF2)7COOH , Aldrich company)
and perfluoro-2 ,5-dimethyl-3, 6–dioxanonanoic acid 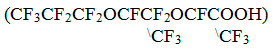
obtained by hydrolysis of hexafluoropropylene oxide trimer (Kirov-Chepetsk
chemical plant; TU 38.03.1.013) has been used as perfluorocarboxylic acids.
Sodium bromide ("analytical grade" in accordance with GOST 4169-76) has been used as a source of bromine.
GLC analysis of the products were carried out on an LHM-8MD chromatograph - Model 5 equipped with thermal conductivity detector, temperature programming 30-200°C (6°C / min); helium was used as a gas-carrier (40 ml/min); columns ( Ø = 3 mm, l = 3 m) with the stationary phase (15% of the masses); SKTFT-50 on a solid support Celite-545 (0,16-0,2 mm at stuffing density of 0.3 g/cm3).
Product rectification was made on a microcolumn ( Ø=8 mm, l = 100 mm) with spiral of 2 mm (Levin nozzle) controlling the composition of fractions by GLC method.
Products of electrosynthesis were identified in accordance with the NMR spectra of 19F recorded on a spectrometer Bruker Spectrospin H-500 (frequency is 470.6 MHz ) in a solution of hexafluorobenzene with the use of deuteroacetone as an internal standard.
Polarization measurements were carried out in galvanodynamic mode as described previously [10], using silver chloride reference electrode.
Preparative synthesis of perfluoro-1-bromooctane
Electrolyzer was a cylindrical vessel with a capacity of up to 30 ml with a magnetic stirrer and a water cooling coil. Current leads of the electrodes, a thermometer, a gas discharge tube with reflux condencer were mounted on the cover.
As an anode a platinum plate with the working surface of 4.4 cm2 have been used. Cathodes were two nickel wire meshes with the working area of 2.2 cm2, placed on both sides of the anode with an interelectrode distance of - 1 cm.
The electrolyte was prepared by mixing 3.09 g (0.03 mole) of sodium bromide and 4.64 g (0.01 mol) of perfluoropelargonic acid with 50% vol. solution of acetonitrile in water. The volume of electrolyte was adjusted to 20 mL. The electrolyte was separated into two layers: a lower aqueous layer was a solution of sodium bromide, which salted out perfluoropelargonic acid dissolving in the upper layer of acetonitrile.
When mixing the layers the emulsion was formed. The electrolysis was carried out with cooling water at 25 ° C, anode current density - 0.3 A/cm2, current strength - 1.32 A until the exhaustion of perfluoropelargonic acid, when the electrolyte in an hour became neutral and homogenized. Bromine liberated on the anode slightly stained the electrolyte. After carrying out of the electrolysis at the bottom there was a liquid product in the form of a heavy organofluoric layer which was separated, washed with 5% Na2CO3, dried over MgSO4 and rectified.
4.40 g of the liquid product was isolated. Boiling point = 143 °C. By NMR spectra of 19F ( Table 1) the product was identified as perfluoro-1-bromoctane (the main substance content was 97% by mass). The admixture (3%) is perfluorohexadecane as the product of the Kolbe synthesis. The yield of perfluoro-1-bromoctane was 91% mole by substance and 37% by current.
Preparative synthesis of perfluoro-2-bromo-2 ,5-dimethyl-3,6-dioxanonane.
20 ml of similarly prepared mixture of 4.96 g (0.01 mole) of perfluoro-2 ,5-dimethyl-3 ,6-dioxanonanoic acid and 3.09 g of NaBr (1,5 gmol/l) was added to 50% solution of acetonitrile and water in the electrolyzer. The electrolysis was carried out with stirring at 25 °C, the current strength - 0.88 A. The anode current density was reduced to 0.2 A/сm2. After an induction period of 1 hour formation of the product was observed, this dripped from the anode surface to the bottom of the electrolyzer forming the third lowest layer.
After 40 minutes of the electrolysis, the electrolyte became neutral and two top layers were homogenizied. The fluoroorganic lower layer was separated and treated similarly as described above. The yield was 5.3 g of almost pure title perfluoro-2-bromo-2 ,5-dimethyl- 3,6-dioksanonane identified in accordance with the NMR spectra of 19F shown in Table 1. Boiling point = 139 °C at 740 mm Hg. The dimerization product content according to the Kolbe synthesis did not exceed 1% by weight. The yield of perfluoro-2-bromo-2 ,5-dimethyl-3,6-dioxanonan was 99 % by substance and 36% by current.
EXPERIMENTAL RESULTS AND DISCUSSION
Polarization measurements
Figure 1 shows the anodic polarization curves of platinum in water-acetonitrile 0.5 M solutions of perfluoro-2 ,5-dimethyl-3 ,6-dioxanonanic acid recorded on the phone of 1.5 M sodium bromide in various ratios of medium components.
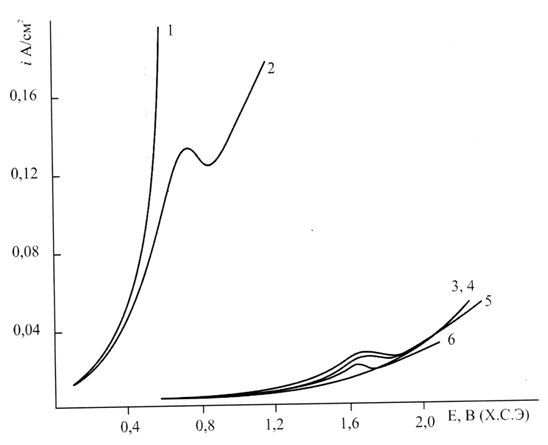
Fig. 1. Polarization curves of Pt-anode in mixtures of CN3CN: H2O on the background
of 1.5 M:
1 - 30:70% by vol. 2 - 50:50% by vol., 3-6 in 0.5 M solutions of |
3-30:70 3% by vol. 5 - 50:50% by vol., 4 and 6 - 90:10% by vol. 6 - in the absence of NaBr
Curves 1 and 2 were recorded in the absence of perfluorocarboxylic acid in the acetonitrile-water mixture that contained 30 and 50% by volume of acetonitrile. The curves show a rapid growth of the polarization, indicating the discharge of bromine-ions in the potential range of 0.6-0.8, and their adsorption on the anode surface. Then bromine-radicals are desorbed as dibromide-molecular.
Curves 3-5 in in the acetonitrile-water mixture that contained 30, 50 and 90 % by volume of acetonitrile show a significant increase in the polarization of the anode in the presence of perfluoro-2 ,5-dimethyl-3 ,6-dioxanonanoic acid . It was apparently associated with the displacement of water from the double-layer by anions of perfluorocarboxylic acid, subsequent discharge of the acid on the anode surface occupied by bromine radicals and the electrochemical desorption with the formation of perfluoro-2-bromo-5-methyl-3 ,6-dioxanonane.
Polarization curve 6 in the absence of sodium bromide corresponds to the electrochemical desorption of perfluorodioxaalkyl radicals with the formation of dimers according to the Kolbe synthesis.
Polarization measurements at least do not contradict the possibility of discharge of the anions of perfluorocarboxylic acids on the anode surface covered by adsorbed bromine radicals.
Thus, the adsorption of a halogen radical competing with the adsorption of perfluorocarboxylic radicals and perfluoroalkyl radicals prevents the electrochemical desorption of perfluoroalkyl radicals in the Kolbe synthesis.
Preparative electrolysis
As shown earlier [5,6] the optimal yield of perfluoropolyethers under anodic condensation of perfluorooxaalkylcarbon acids in the Kolbe synthesis is obtained with volume ratio CH3CN:H2O as 90:10.
To implement the above-mentioned competing process leading to the electrosynthesis of perfluoralkylbromide as the title product, it was necessary to find optimal conditions for preparative electrolysis, i. e. to provide the increase in adsorbed halogen concentration on the anode and the reduction of adsorption of perfluoralkyl carboxylate radicals on the anode.
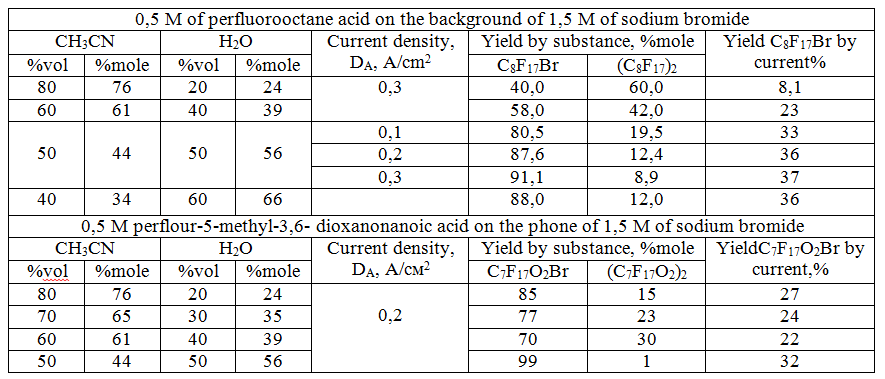
We investigated the dependence of the perfluoralkylbromide yield on the current density and the composition of water-acetonitrile mixtures in preparative electrolysis (0.5 M solution of perfluorohexanoic acid and perfluoro-2,5-dimethyl-3 ,6- dioxanonanoic acids) in the presence of 1.5 M sodium bromide on the platinum anode with the nickel cathode. The results are shown in Table 2.
Obviously, the maximum perfluoroacyl bromide yields were obtained with ratio of acetonitrile and water significantly different from the optimal implementation of the Koble synthesis 90:10 . In this case, the optimum is close to an equal volume ratio 50:50, providing perfluoralkylbromide yields above 90 mol %, whereas the yields of dimerization products in the Kolbe synthesis did not exceed 10 mol %.
The increase of the volume fraction of acetonitrile in the medium above 50% vol. causes 1) the increase of the solubility of perfluorocarboxylic acids in the mixture and 2) the adsorption of their anions on the anode during high anodic polarization, providing their Kolbe electrosynthesis with the growth of dimers yield at 90 volume % CH3CN [5,6].
The comparison of the dependence of perfluoro-2-bromo-5-methyl-3 ,6-dioksanonane yields ( by substance and by current ) on the current density and medium composition with polarization measurements apparently confirm the idea that the limiting stage of the process is the adsorption of bromine radicals formed as a result of bromine anion discharge at significantly lower anodic potentials.
The reduction of acetonitrile content in the medium below 40 % by volume causes the decrease of solubility of perfluorocarboxylic acids in the mixture accompanied by micelle formation. It causes strong foaming of the electrolyte that is connected with adsorbtion of acid-anions that are emulgators in this case on the border electrolyte - electrolyze gases.
Salting-out effect of sodium bromide causes the formation of an emulsion with the formation of micellar structures containing acetonitrile and perfluorocarboxylic acid, stabilized on the border of the micelles with an aqueous solution of sodium bromide by anions of perfluorocarboxylic acids.
The data in Table 2 show that perfluoroalkyl bromides yield in the electrolysis performed in perfluoro-2,5-dimethyl-3,6-dioxanonane acid is much higher and less dependent on acetonitrile-water ratio than in the case with perfluorononan acid. It is likely to be associated with the difficulties in adsorption of a perfluorocarboxylate radical due to the presence of trifluoromethyl group in a-position to the carboxyl group. Free rotation of the perfluoroalkyl fragments around ester bond formation is likely to affect. It provides a more dense packing of perfluoroalkyl fragment during the adsorption process at the interface.
Conclusions
1. Preparative electrolysis of solutions of perfluorocarboxylic acids on the Pt-anode on 1.5 M sodium bromide as a phone in the medium of acetonitrile- water mixture indicates the possibility of electrosynthesis of various perfluoroalkylbromides with the substance yield of 90 mol %.
2. Polarization curves recorded on the Pt-anode in the medium of acetonitrile- water mixture in the presence of 1.5 M sodium bromide as a phone are more likely to confirm confirms the mechanism of electrochemical desorption of perfluoroalkylbromides as a result of the anion discharge of perfluorocarboxylic acids on the anode surface occupied by adsorbed bromine radicals.
Table 1. NMR spectra of 19F of perfluoroalkylbromides.
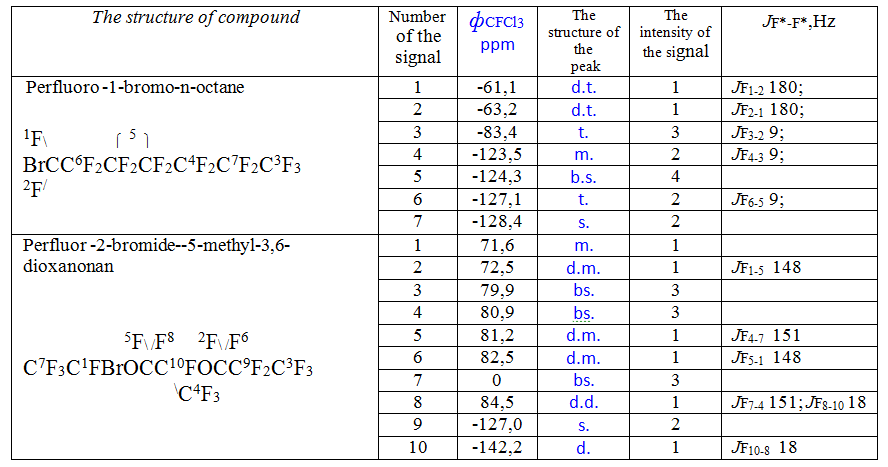
Table 2. Dependence of product yields on medium composition, current density and the structure of perfluorocarboxylic acids in preparative electrolysis on Pt-anode of 0.5 M solution on the background of 1.5 M .
References
2. Miller M.L., Stinnet J.D., Clark S.C. Ir. // J. Reticuliendothel. Soc., 1980, V. 27, P. 81-97.
3. G.D.Orlov, V.A.Saraev // «Soedineniya ftora. Khimiya, tekhnologiya, primenenie», Sbor. nauch. tr. RNC «Prikladnaya khimiya», 2009 g., s.41.
4. A.I.Levin, S.V.Sokolov, O.N.Chechina, K.N.Bil'dinov // ZhOKh, 1969, 39, 440
5. N.V.Peganova, A.A.Ludiikainen, V.A.Matalin, T.V.Mikchailova, N.V.Puzanova, V.V.Loginova, G.I.Kaurova // Fluorine Notes, 2009 N 6 (67)
6. V.F.Cherstkov, V.A.Grinberg, S.R.Sterlin, Yu.V.Vasil'ev, L.S.German // Izv. AN SSSR, ser. khim. 1990, №10, 2448
7. V.A.Grinberg, V.F.Cherstkov, S.R.Sterlin, Yu.V.Vasil'ev, L.S.German // Izv. AN SSSR, ser. khim., 1990, №8, s. 1841
8. Patent SU №1141706, 1984, MKI «Sposob polucheniya perftoroktilboromida»
9. «Organikum». Praktikum po organicheskoj khimii, 1979, «Mir», Moskva, T. 2, s. 356.
10. Chechina O.N., Berenblit V.V., Levin A.I. // ZhPKh, 1999, T.72, V. 3, s. 410-415.
Recommended for publication by prof. Alexandr Y. Zapevalov
Fluorine Notes, 2012, 81, 5-6
