Received: Febrгary, 2012
Fluorine Notes, 2012, 81, 3-4
Interaction of 2-substituted 7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinoline-4-carboxylic acids with pentafluorphenyl hydrazine. Synthesis of 5-substituted 8,8-dimethyl-2-pentafluorphenyl-3,7,8,9-tetrahydro-2H-pyrido[4,3,2-de]cinnolin-3-ones
D.A. Rudenko, V.I. Karmanov1, P.T. Pavlov, M.I. Vahrin, S.N. Shurov, L. N. Bazhenova2
Perm State University, Organic Chemistry Department, 614990 Perm, Bukirev st., 15, tel.: +7(342)239-66-12
e-mail: seshurov@yandex.ru
1Institute of Technical Chemistry of UB RAS, 614013 Perm, Academica Koroleva st., 3
2Institute of Organic Synthesis of UB RAS, 620041, Ekaterinburg, S. Kovalevskya st., 22/ Academicheskya st.,20
Abstract: 2-Substituted 7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinoline-4-carboxylic acids react with pentafluorphenyl hydrazine to give 5-substituted 8,8-dimethyl-2-pentafluorphenyl-3,7,8,9-tetradihydro-2H-pyrido[4,3,2-de]cinnolin-3-ones. The structures of synthesized compounds were based on 1H and 19F NMR spectroscopic data.
Keywords: 2-substituted 7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinoline-4-carboxylic acids, pentafluorphenyl hydrazine, 5-substituted 8,8-dimethyl-2-pentafluorphenyl-3,7,8,9-tetradihydro-2H-pyrido[4,3,2-de]cinnolin-3-ones.
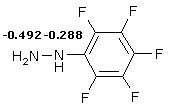
As known ethers of 2-substituted 5-oxo-5,6,7,8-tetrahydroquinoline-4-carboxylic acids react with hydrazine to afford 5-substituted 7,8,9-tetradihydro-2H-pyrido[4,3,2-de]cinnolin-3-ones [1]. Concordant results were obtained in the research on reaction between 2-substituted 7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinoline-4-carboxylic acids and hydrazine and its ethyl- and phenyl- derivatives [2]. Formation of pyridazine fragment is caused by participation in reaction of both hydrazine and its monoderivatives nitrogen atoms. The behavior of pentafluorphenyl hydrazine in such reaction hasn’t been explored. The interest to this substance is conditioned by considerable difference in nucleophilicity of nitrogen atoms N1 and N2 in its molecule. It seems only one nitrogen atom N2 able to take part in interaction. This assumption is based on nonobservational quantum-chemical calculations of Lowdin's total nitrogen atomic charges in pentafluorphenyl hydrazine molecule.
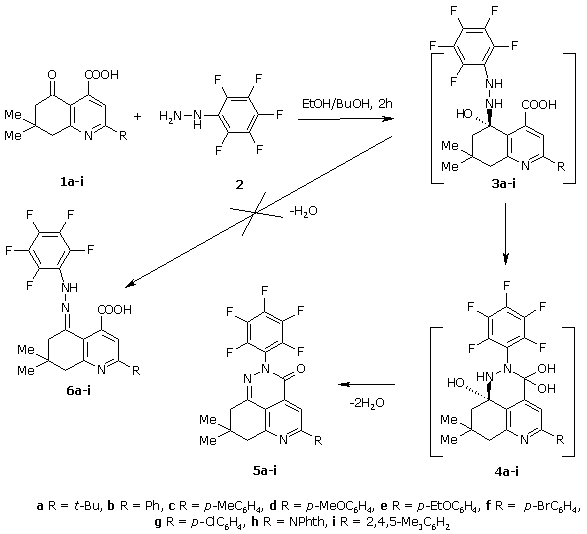
The reaction between equimolar quantities of 2-substituted 7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinoline-4-carboxylic acids 1a-i and pentafluorphenyl hydrazine (2) leads to 5-substituted 8,8-dimethyl-2-pentafluorphenyl-3,7,8,9-tetradihydro-2H-pyrido[4,3,2-de]cinnolin-3-ones 5a-i in good yields.
Synthesized pyrido[4,3,2-de]cinnolin-3-ones 5a-i are colorless crystalline substances. Yields, melting points and element analyses data are summarized in table 1.
Table 1. Yields, melting points and element analyses data.
| Compound | Gross-formula | Found, % Calcl.,% |
MP °C (solv.) |
Yield, % | |||
| C | H | N | F | ||||
| 5a |
C22H20F5N3O |
60.41 60.41 |
4.52 4.61 |
9.47 |
21.47 21.82 |
98-99 (EtOH) |
75 |
| 5b |
C24H16F5N3O |
62.87 63.02 |
3.60 3.53 |
9.16 9.19 |
20.77 20.77 |
174-175 (BuOH) |
72 |
| 5c |
C25H18F5N3O |
63.92 63.69 |
3.81 3.85 |
8.91 8.91 |
20.08 20.15 |
195-196 (BuOH) |
72 |
| 5d |
C25H18F5N3O |
61.59 61.60 |
3.50 3.72 |
8.58 8.62 |
19.50 19.49 |
174-175 (EtOH) |
83 |
| 5e |
C26H20F5N3O2 |
62.46 62.28 |
3.89 4.02 |
8.48 8.38 |
18.85 18.94 |
133-134 (EtOH) |
68 |
| 5f |
C24H15BrF5N3O |
53.77 53.75 |
2.88 2.82 |
7.77 7.84 |
17.67 17.71 |
211-212 (BuOH) |
69 |
| 5g |
C24H15ClF5N3O |
58.74 58.61 |
3.26 3.07 |
8.54 8.54 |
19.61 19.31 |
194-195 (BuOH) |
67 |
| 5h |
C28H18F5N3O |
66.41 66.27 |
3.61 3.58 |
8.28 8.28 |
18.75 18.72 |
163-164 (BuOH) |
63 |
| 5i |
C27H22F5N3O |
65.03 64.93 |
4.28 4.44 |
8.34 8.41 |
19.03 19.02 |
192-193 (BuOH) |
85 |
The scheme of formation compounds 5a-i includes addition of pentafluorphenyl hydrazine molecule (2) to carbon atom C5 of initial acid 1a-i and intermediate 3a-i cyclization by means of intramolecular nucleophilic attack of nitrogen atom connected with pentafluorphenyl radical at carboxylic carbon atom. Elimination of two water molecules leads to products 5a-i. Probably intramolecular nucleophilic attack is more preferable in comparison with dehydration followed by formation of corresponding pentafluorphenyl hydrazones 6a-i.
The structures of products were determined on IR, 1H and 19F NMR spectroscopic data. IR spectrum shows characteristic band of C3=O stretching vibrations in 1681-1695 cm-1 region, C-F vibrations in 993-1005 cm-1 and intensive band 1586-1596 cm-1 attributed either to C=N or C=C bond stretching vibrations. 1H NMR spectrum demonstrates signals of methyl protons at 1.14-1.21 ppm, methylen C7H2 and C9H2 protons at 3.03-3.17 and 2.82-2.91 ppm respectively, C4H proton in weak field at 7.64-8.44 ppm and also signals of substitute's protons.
Table 2. IR and 1H NMR spectra data.
| Conpound | IR spectra, ν, cm-1 | 1H NMR spectra, δ, ppm (J, Hz) | ||||||
C=C + C=N |
C3=O |
C–F |
6H, 2CH3 (c) |
2H, C7H2 (c) |
2H, C9H2 (c) |
1H, C4H (c) |
R-signal |
|
| 5a | 1596 | 1687 | 998 | 1.14 | 3.09 | 2.82 | 7.97 | 1.44 (9H, s, C(CH3)3) |
| 5b | 1591 | 1681 | 993 | 1.17 | 3.17 | 2.85 | 8.43 | 7.49-7.59 (3H, m, C6H5) 8.27-8.30 (2H, m, C6H5) |
| 5c | 1587 | 1688 | 1002 | 1.17 | 3.16 | 2.84 | 8.41 | 2.41 (3H, s, CH3) 7.37 (2H, d, J = 8.0, H Ar) 8.20 (2H, d, J = 8.0, H Ar) |
| 5d | 1591 | 1684 | 1003 | 1.17 | 3.13 | 2.83 | 8.35 | 3.89 (3H, s, OCH3) 7.10 (2H, s, J = 9.0, H Ar) 8.27 (2H, s, J = 9.0, H Ar) |
| 5e | 1586 | 1689 | 1003 | 1.17 | 3.13 | 2.82 | 8.35 | 1.40 (3H, t, J = 7.0, OCH2CH3) 4.15 (2H, q, J = 7.0, OCH2CH3) 7.08 (2H, d, J = 9.0, H Ar) 8.26 (2H, d, J = 9.0, H Ar) |
| 5f | 1596 | 1688 | 1004 | 1.17 | 3.17 | 2.85 | 8.44 | 7.72 (2H, d, J = 9.0, H Ar) 8.25 (2H, d, J = 9.0, H Ar) |
| 5g | 1598 | 1687 | 1005 | 1.17 | 3.16 | 2.85 | 8.43 | 7.57 (2H, d, J = 9.0, H Ar) 8.31 (2H, d, J = 9.0, H Ar) |
| 5h | 1592 | 1689 | 999 | 1.21 | 3.03 | 2.91 | 7.64 | 7.82-7.50 (4H, m, C10H7) 8.23-8.02 (3H, m, C10H7) |
| 5i | 1596 | 1695 | 998 | 1.17 | 3.14 | 2.86 | 8.05 | 2.29 (6H, s, 2CH3) 2.39 (3H, s, CH3) 7.11 (1H, s, C6H2(CH3)3) 7.37 (1H, s C6H2(CH3)3) |
19F NMR spectrum contains characteristic signals of pentafluorphenyl group (table 3).
Table 3. 19F NMR spectra data.
|
Product |
1 NMR spectra, δ, ppm (J, Hz) |
||
|
Fp (t) |
Fo (d) |
Fm (set of signals) |
|
|
5a |
-78.36 |
-70.08 |
-87.35 |
|
5b |
-78.28 |
-69.86 |
-87.25 |
|
5c |
-78.32 |
-69.91 |
-87.28 |
|
5d |
-78.39 |
-69.90 |
-87.32 |
|
5e |
-78.39 |
-69.88 |
-87.31 |
|
5f |
-78.20 |
-69.81 |
-87.22 |
|
5g |
-78.21 |
-69.80 |
-87.22 |
|
5h |
-78.63 |
-70.34 |
-87.67 |
|
5i |
-74.54 |
-67.80 |
-83.91 |
In conclusion, an efficient procedure for the synthesis of 5-substituted 8,8-dimethyl-2-pentafluorphenyl-3,7,8,9-tetradihydro-2H-pyrido[4,3,2-de]cinnolin-3-ones from 2-substituted 7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinoline-4-carboxylic acids and pentafluorphenyl hydrazine is developed. It is found that nature of substituent at second position in initial acids doesn’t effect at yield.
Experimental
IR spectra were recorded on IFS 66ps "Bruker" FT-spectrometer in mineral oil suspension. 1Í and 19F NMR spectra were obtained for solution in acetone-d6 with HMDSO (δ = 0.059 ppm) and triflouracetic acid (δ = 0.000 ppm) as internal standards using Varian MERCURY+300 spectrometer at 300ÌHz and 282MHz correspondingly. Element analyses were carried out using certified methodolodes: MVI 88–16358–124–2011 "Determination of carbon, hydrogen and nitrogen mass fraction in organic compounds" at automatic "CHN" ÐÅ-2400, ñ.II analyzer (Perkin Elmer Instruments, USA). F-analysis was done using MVI # 88–16358–95–2009 "Determination of flour mass fraction in organic compounds using spectrophotometric method" at spectrophotometer "Specord 200" PC ("Analitic Jena AG" Germany).
Quantum-chemical calculations were carried out with program package Firefly [3] on personal computer Toshiba 400 PORTEGE.
5-Substituted 8,8-dimethyl-2-pentafluorphenyl-3,7,8,9-tetradihydro-2H-pyrido[4,3,2-de]cinnolin-3-ones 5a-i (general procedure). A mixture of corresponding 2-substituted 7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinoline-4-carboxylic acid 1a-i (3 mmol) and pentafluorphenyl hydrazine (2) (0.6 g, 3 mmol) is heated at reflux in ethyl or butyl alcohol (30 ml) for 2 h. After cooling the reaction mixture crude product is filtered and recrystallised from ethyl or butyl alcohol.
References
- Mulamba T., El Boukili-Garre R., Seraphin D., Noe E., Charlet-Fagnere C., Henin J., Laronze J., Sapi J., Barret R., Laronze J-Y., Levy J., Heterocycles, 41, 29 (1995).
- Shurov S. N., Rudenko D. A., Karmanov V. I., Naymushina Ya. A. Interaction of 2-aryl-7,7-dimethyl-5-oxo-5,6,7,8-tetrahydroquinoline-4-carboxylic acids with hydrazines. Synthesis of 5-aryl-8,8-dimethyl-8,9-dihydro-2Í-pyrido[4,3,2-de]cinnolin-3(7Í)-ones, in Abstracts of III International Conference on Heteroceclic Chemistry, in Honor of the late Professor A.N. Kost's 95th Anniversary, Moscow, Russia, 2010, p. 175.
- Granovsky À. À. Firefly version 7.1.G. URL: http://classic.chem.msu.su/gran/firefly/index.html (address date: 02.11.2011).
Recommended for publication by Prof. A. Zapevalov
Fluorine Notes, 2012, 81, 3-4
