Received: January, 2012
Fluorine Notes, 2012, 80, 5-6
Antifrictional properties of fluorine-containing polyol esters
T.I. Gorbunova a*, D.N. Bazhina, A.Ya. Zapevalova , L.G. Korshunovb, I.V. Beketovc, V.I. Saloutina
a I.Ya. Postovsky Institute of Organic Synthesis, Ural Branch, Russian Academy of
Sciences, 620990, Ekaterinburg, Kovalevskoy, 22
e-mail: gorbunova@ios.uran.ru
b Institute of Metal Physics, Ural Branch, Russian Academy of Sciences, 620990, Ekaterinburg,
Kovalevskoy, 18
e-mail: korshunov@imp.uran.ru
c Institute of Electrophysics, Ural Branch, Russian Academy of Sciences, 620016, Ekaterinburg,
Amundsena, 106
e-mail: beketov@iep.uran.ru
Abstract:Equilibrium mixtures of fluorine-containing polyol-based esters were synthesized using trimethylolpropane (TMP), 2,2-dimethyl-1,3-propanediol (neopentyl glycol, NPG) and polyethylenglycol 1000 (PEG-1000). The obtained products were used as additives (1 wt%) to petroleum-based oil. It has been found that the greatest contribution to the improvement of antifrictional properties of petroleum-based oil is made by an additive of the equilibrium mixtures of the esters with polyfluorooxaalkyl substituent [C3F7OCF(CF3)CF2OCF(CF3)]. Some aspects of possible adsorption of the fluorine-containing polyol-based esters on a steel surface are discussed in the paper. All equilibrium mixtures of the synthesized esters are perspective stabilizers of copper nano-powder (1 wt%) suspensions in the petroleum-based oil. The obtained suspensions improve antiwear properties of austenitic chromium-containing steel.
Keywords: perfluoropolyalkyl ethers and esters, lubricant additives, friction coefficient, wear intensity, copper nano-powder,
1 Introduction
It is known that fluorine-containing organic compounds that have improved viscosity-temperature parameters in comparison with their non-fluorinated analogues as well as higher thermal stability and resistance to oxidation, possess low friction coefficients and show excellent antiwear properties under friction. Well-known trade marks of perfluoropolyethers (PFPE), such as Krytox, Fomblin and Demnum, have the greatest popularity among fluorine-containing fluid lubricants. Depending on the service conditions they can be supplied by various end groups, functional and non-functional. For example, Fomblin Z03 contains non-functional end groups OCF3; Fomblin Z-DOL and Demnum SA have reactive CH2OH-groups; Fomblin Z-DIAC contains carboxy-group COOH [1]. However, the use of PFPE is limited since. It has been shown that they undergo decomposition by a disproportionation reaction mechanism to form acid fluorides (COF-group) when heated to nearly 300°C under friction [2,3]. The formation of the compounds with the high reactive ability influences the friction process in two ways. Some formed products upon the disproportionation reaction are spent on the formation of metal salts on the friction surface [4]. It promotes formation of metal oxides [5] and improves the tribological process due to monomolecular salt layer strongly connected with a surface from a pure metal. The rest of the allocated products is exposed to further oxidative processes as a result of which the antifrictional parameters of PFPE used worsen greatly. Both the molecular weight of the ether and the nature of end groups influence the thermal decomposition [6]. Consequently, PFPE need antioxidants additions, and friction surfaces when PFPE are used should be made of corrosion resistant materials [3,5,7-9]. Application of PFPE on metal surfaces made of a steel and aluminum is preferable [8,10-13].
The use of PFPE as additives to the petroleum-based oils would be logical. But the fact is that PFPE possess extremely oleophobic structures and do not mix up with oils of the hydrocarbonic nature. Consequently, when employing fluorine-containing compounds as additives to mineral oils the substrates with hydrocarbon oleophilic substituent are necessary.
The positive effect of the oil-additive use depends on the nature of the film formed on the friction surface due to adsorbtion, chemosorbtion and tribochemical reactions.
Taking this into consideration, we consider esters based on fluorocarbon acids and polyols to be very perspective materials. The purpose of this paper is synthesis of such objects and study of their antifrictional properties.
2 Experimental
2.1 Materials
Reagents.
Polyols. Trimethylolpropane (2-ethyl-2-(hydroxymethyl)-1,3-propanediol, TMP) (Alfa
Aesar Co.) at a purity of 98%; 2,2-dimethyl-1,3-propanediol (neopentyl glycol, NPG)
(Alfa Aesar Co.) at a purity of 97%; polyethylenglycol 1000 (PEG-1000) (Merck Co.).
Perfluorocarbon acids and acid fluoride. Perfluoropentanoic, perfluoroheptanoic,
ω-chloroperfluorononanoic acids (C4F9COOH, C6F13COOH,
ClC8F16COOH) and 2,4,4,5,7,7,8,8,9,9-undecafluoro-2,5-bis(trifluoromethyl)-3,6-dioxanonanoyl
fluoride (AF) (C3F7OCF(CF3)CF2OCF(CF3)COF)
at a purity of 98%.
Petroleum-based
oil.
In the research oil with a viscosity class 32 (DIN 51 516, ISO 3448-75) is used.
Steel.
Austenitic steel A2 (AISI304) is used as a friction surface. The steel consists
of C 0.12%, Cr 17-19%, Ni 8-10%, Si 0.8%, Mn 2.0%, S 0.02%, P 0.035%. Such choice of steel
creates tough conditions of boundary friction owing to the raised plasticity of the metal
surface. It leads to the destruction of surface films at lower temperatures and increases
the steel gripping power [10].
Copper nano-powder.
The powder was produced by the electrical explosion wire method
(EEW) [14]. The diameter of the copper wire was 0.4 mm and the wire length 90 mm. The inductance
of the discharge circuit was 0.4 μH. The value of the capacitor bank was 4.8 μF; the charging
voltage was 30 kV that provided the necessary overheat of metal. Copper’s specific energy
of sublimation is 47.8 J/mm3. The wire was fed into the installation continuously
and exploded in argon at a pressure of 0.12 MPa and a frequency of 0.5 Hz. The powder was
analyzed using BET (Micromeritics Tristar, USA) and XRD (D8 Discover X-ray Diffractometer
with Gadds, Bruker AXS, Germany) methods. According to the XRD results the copper powder
after one-year-storage contained 22 wt% of Cu2O. Transmission electron microscopy
(TEM) examinations showеd the diameters of the prepared Cu nanoparticles (Figure
1). The average size of the copper’s coherent-scattering region was 56 nm.

Fig. 1. TEM micrograph of the copper nano-powder
2.2 Synthesis of fluorocontaining esters
The fluorocarbon acid (C4F9COOH or C6F13COOH or ClC8F16COOH) in amount of 0.05 mol was added to melted polyol (TMP or NPG or PEG-1000) in amount of 0.05 mol. Two drops of sulfuric acid (H2SO4conc.) were added. The mixture was stirred for nearly 4 hours at temperature 100оC. After that the soda solution (10 wt %) was brought to achieve the neutralization (pH 7).
When AF (C3F7OCF(CF3)CF2OCF(CF3)COF) was used, the sulfuric acid was not required.
Then in case of TMP and NPG esters the organic layer was separated, washed out several times with water and dried with CaCl2. In case of PEG-1000 esters the reaction mixture was extracted with chloroform (CHCl3, 2x50 ml, 1x30 ml), the organic layer was washed out with water and dried with CaCl2. After that the chloroform was removed by distillation.
2.3 Analysis
IR spectra were registered on Perkin–Elmer model Spectrum One B spectrometer.
NMR spectra (1H and 19F) were recorded on Bruker DRX-400 400 MHz spectrometer, operating frequencies - 400.1 MHz (1H) and 376.5 MHz (19F), internal standards - Me4Si and C6F6, respectively. 19F NMR chemical shifts were reported relative to hexafluorobenzene at 0 ppm. Samples were dissolved in CDCl3.
The element analysis was carried out by means of automatic analyzer Perkin–Elmer CHN PE 2400.
Measurements on gas chromatography with mass-spectrometer detector (GC-MC) were made, using Agilent 7890A MS 5975C Inert XL and quartz capillary column HP-5-MS (30 m • 0.26 mm • 0.25 µm). The initial temperature of the column was 60°C (time delay 2 min), the heating was performed with a speed of 10°C/min to 290°C (time delay 20 min). The temperature of the evaporator was 250°C. The gas carrier was helium, split ratio was 1:50. The ionization energy was 70 eV.
2.4 Method of antifrictional research
Research of the values of the friction coefficient (f) and wear intensity (Iw) were carried out on the "finger-plate" scheme under conditions of a sliding friction at room temperature. Finger movement on a plate was reciprocating. Before this the plate was greased by the prepared oil with the synthesized fluorocontaining esters as a lubricant additives (1 wt%).
The finger and plate represent parallelepipeds from the austenitic steel A2 (AISI304) with the same name. The finger size is 7x7x20 mm, the plate size is 60x40x10 mm. The sliding speed of finger along the plate is 0.07 m/sec. The length of the finger’s friction path was 80 m (~ 1000 double strokes of the finger).
The working surfaces of fingers and plates were grinded by hand, class of surface roughness was eight (ISO/R-468). Before the tests the fingers and plates were carefully degreased. Before the starting moving a drop of the prepared oil (100 µl) was put on the plate.
The friction coefficient (f) was determined as the ratio of frictional force (F) to normal loading (N) according to the formula:
f = F / N
The frictional force (F) was measured by means of an elastic element (a steel ring) with strain-gauge transducer fixed on it. Indications were recorded on a tape of an electronic potentiometer. The normal loading (N) was 294 N.
The wear intensity (Iw) of the fingers was calculated according to the formula:
Ih = ∆Q / ρ•L•S ,
∆Q – weight loss of a finger, g; ρ – density of a finger material, g/cm3; L – friction path, cm, S – geometrical contact area, cm2.
Weight losses (∆Q) were defined by weighing the fingers degreased after the tests on analytic balance with precision of 0.0001 g.
3 Results and discussion
3.1 Synthesis
It was shown before that favorable conditions for the synthesis
of an incomplete ester (monoester A) based on PEG-600 and oleic acid are the 1:4 molar
ratios of oleic acid to PEG-600, use of a catalyst (zeolite-β, Nafion or p-toluene
sulphonic acid) at temperature 130°C [15]. At the 1:1 molar ratios of oleic acid to PEG-600
in the same conditions the complete ester (diester B) is formed as a by-product (Scheme 1).
Scheme 1
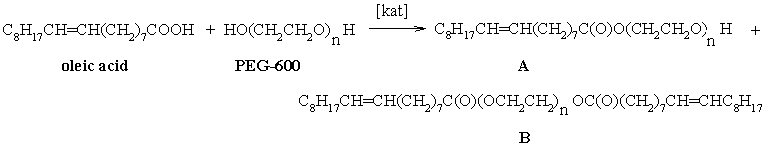
For our work it is important that not all HO-groups of polyols are esterificated by perfluorocarbon acids, but only their part. The presence of hydroxyl groups in molecules of the incomplete esters provides esters with polar properties, which is shown in high affinities of the esters for different surfaces [16-18]. Besides, for the esters based on PEG the additional increase in adhesion is typical due to the presence of ethers’ oxygen in a polymeric chain. Using a classical technique of esterification with mineral acid catalyst we intentionally introduced into a reactionary mixture a lack of the acid with respect to the polyols. The molar ratios of the perfluorocarbon acids to the polyols were 1:1. This procedure was applied to obtain the equilibrium mixtures of the complete and incomplete esters (Scheme 2).
Scheme 2
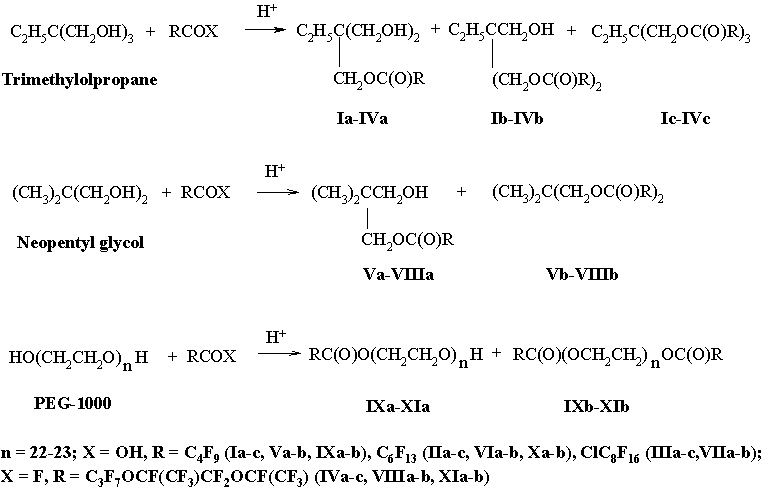
From well-known structural analogues of synthesized esters one can mention complete esters based on TMP and heptanoic acid C2H5C(CH2OC(O)C6H13)3, the trade name is MIL-L-7808J. This ester has proved thermostable lubricant for gas turbines and is utilized today at rather high temperatures (nearly 300°C) [19,20]. The TMP triester of 10-undecenoic acid is found for high performance lubricants [21]. Besides, incomplete esters based on PEG, TMP, NPG, pentaerythritol and dicarbon acids are semi-products for production of materials which improve the dispersant-viscosity parameters of lubricants [22].
Even a mere mechanical mixing of polyols with carbon acids leads to synergistic effects in the process of improving antifrictional properties of lubricants as it has been shown in case of higher PEGs [23]. All these facts predict positive influence of new compounds (I-XI) on antifrictional properties of the oils with additives made from them.
3.2 Determination
of mixture components
The quantitative contents of esters (I-VIII) were established by GC-MC by means of the method
of internal normalization [24], compounds (IX-XI) – by means of NMR (1H, 19F)
and elemental analysis (Table 1). In Figure
2 the spectra of the compounds (IXa,b) are presented.
Table 1 The content of individual esters in mixtures.
|
# |
R |
The content of polyols’ derivatives in mixtures, % |
||||||
|
Trimethylolpropane |
Neopentyl glycol |
PEG-1000 |
||||||
|
mono- |
di- |
tri- |
mono- |
di- |
mono- |
di- |
||
|
1. 2. 3. 4. |
C4F9 C6F13 ClC8F16 C3F7OCF(CF3)CF2O- CF(CF3) |
26.6 6.2 15.3 31.2 |
54.6 64.4 58.7 48.9 |
17.3 29.4 25.6 19.3 |
33.6 39.1 40.5 42.3 |
65.3 60.9 51.6 55.8 |
6.0 10.0 - 8.0 |
94.0 90.0 - 92.0 |
Data of the analysis of PEG-1000 derivatives.
Mixture of monoester (IXa) and diester (IXb). The product was isolated in 76% yield. It represents yellowish amorphous substance. IR, ν, cm-1: 3474 (OH), 2887 (C-H), 1782 (C=O), 1359, 1342, 1319, 1280, 1239, 1206, 1113 (C-O-C, C-F). 1H NMR (CDCl3, δ, ppm): 3.73 (m, (OCH2CH2)n), 4.53 (m, 1H, OH). 19F NMR (CDCl3, δ, ppm, J/Hz): 35.36 (m, 2F, CF3CF2), 38.22 (m, 2F, CF3CF2CF2), 43.13 (m, 2F, CF2C(O)), 80.91 (tm, 3F, CF3, JF-F 9.6). Anal. calcd for C49H89F9O24: C, 44.97; H, 7.10; F, 14.44. Found: C, 47.72; H, 7.27; F, 13.86.
Mixture of monoester (Xa) and diester (Xb). The product was isolated in 78% yield. It represents white amorphous substance. IR, ν, cm-1: 3472 (OH), 2885 (C-H), 1781 (C=O), 1358, 1343, 1324, 1280, 1241, 1209, 1146, 1113 (C-O-C, C-F). 1H NMR (CDCl3, δ, ppm): 3.76 (m, (OCH2CH2)n), 4.54 (m, 1H, OH). 19F NMR (CDCl3, δ, ppm, J/Hz): 35.69 (m, 2F, CF3CF2), 39.02 (m, 2F, CF3CF2CF2), 39.17 (m, 2F, CF2CF2C(O)), 39.98 (m, 2F, CF3(CF2)2CF2), 43.39 (m, 2F, CF2C(O)), 81.08 (t, 3F, CF3, JF-F 9.5). Anal. calcd for C51H89F13O24: C, 43.18; H, 6.79; F, 20.56. Found: C, 45.95; H, 6.73; F, 18.52.
Mixture of monoester (XIa) and diester (XIb). The product was isolated in 73% yield. It represents white amorphous substance. IR, ν, cm-1: 3470 (OH), 2883 (C-H), 1783 (C=O), 1356, 1341, 1323, 1282, 1247, 1200, 1159, 1118 (C-O-C, C-F). 1H NMR (CDCl3, δ, ppm): 3.68 (m, (OCH2CH2)n), 4.53 (m, 1H, OH). 19F NMR (CDCl3, δ, ppm, J/Hz): 16.56 (m, 1F, C3F7OCF), 30.22 (dm, 1F, CFC(O), JF-F 20.1), 32.15 (m, 2F, CF3CF2), 77.40 (m, 1F, CFFOCF(CF3)C(O)), 79.58 (m, 3F, CF(CF3)C(O)), 80.13 (m, 2F, CF3CF2CF2), 80.46 (m, 3F, CF(CF3)CF2O), 81.76 (m, 3F, CF3CF2CF2), 82.76 (dm, 1F, CFFOCF(CF3)C(O), JF-F 146.2). Anal. calcd for C53H89F17O26: C, 41.44; H, 6.31; F, 24.09. Found: C, 43.45; H, 6.12; F, 22.04.
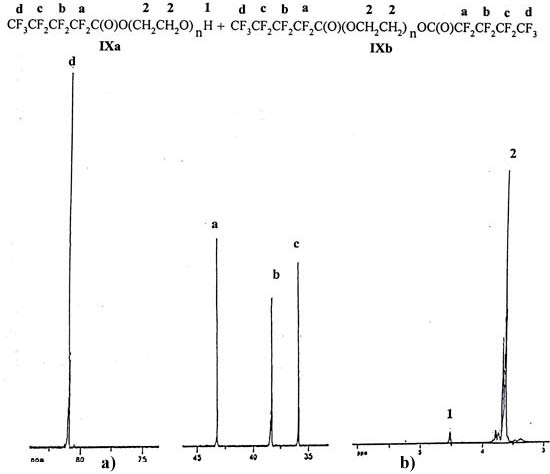
Fig. 2 19F NMR (а) and 1H NMR (b) spectra of ester mixture (IXa) and (IXb.)
3.3 Antifrictional
properties.
In this study it has been shown that the synthesized ester mixtures based
on TMP, NPG and PEG-1000 are well-combined with petroleum-based oil upon mechanical mixing.
It is not the case with the mixtures based on AF (IV,VIII,XI), where ultrasonic dispersion
for formation of their emulsion in the oil of the hydrocarbonic nature is required. In
this study the ultrasonic disperser was applied for two minutes (operating frequency
22 ±10% kHz).
For studying of antifrictional properties of obtained the esters the mixtures based on the oil (99 wt%) and compounds (I-XI) (1 wt%) were prepared and their friction coefficients (f) and wear intensity (Iw) were measured. Four parallel measurements were made for each f and Iw values, the standard deviation is no more than 3 %. The obtained data are presented in Table 2.
The analysis of the data in Table 2 shows that with an increase in length of fluoroalkyl chain in the fluorine-containing ester molecules based on same polyol the antifrictional characteristics of all lubricant mixtures improve. It is in accordance with the data of papers [25,26], where it was found out that within one family of lubricants during tests in the same conditions the friction coefficient decreases with an increase in the molecular weight a lubricant. The reason for this is higher stability of surface films at higher molecular weight.
Table 2. Antifrictional parameters of lubricants based on petroleum-based oils with additives.
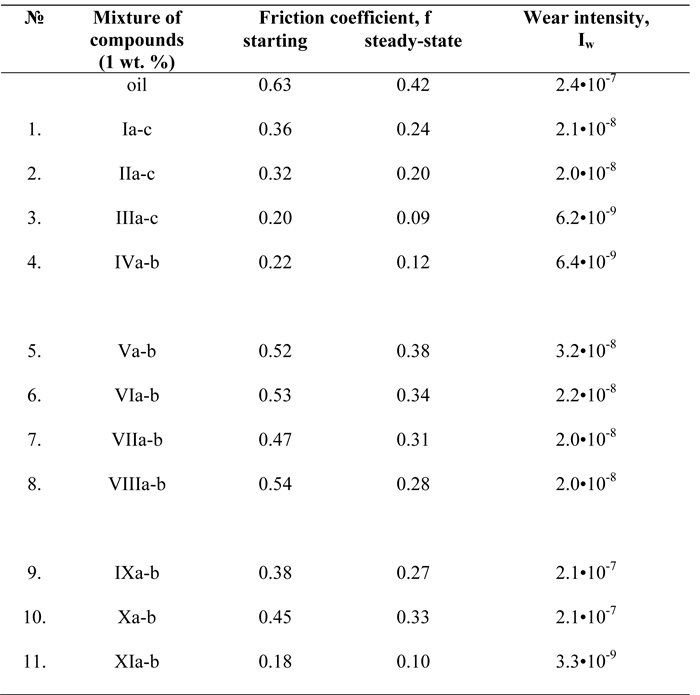
Among the PEG-1000 derivatives (IX-XI) some anomaly is observed (Figure 3). Among them the lubricant with an additive of mixture (XI) possesses the greatest effect of tribological improvement. Steady-state friction coefficient (f) decreased more than four times and wear intensity (Iw) was improved by two orders of magnitude. It is obvious that here the dominant role is played by the combination of several factors:
- antifrictional tests are made at room temperature and relatively low speed of reciprocating movement of a finger on a plate. Consequently, in the given conditions there is no substantial increase in the temperature of friction surfaces and it is possible to neglect thermal disproportionation of the investigated additives;
- adsorption of mixture molecules (XI) on a metal surface is considerably higher than in the case of the other esters. The adhesion fixing of additives (XIa-b) occurs by means of two parts: long chain alkylether fragment and perfluorooxaalkyl radical [2,11] (Figure 4). The esters based on TMP and NPG with perfluoroalkyl substituents (I-III,V-VII) are adsorbed by means of HO-groups only and the esters (IX,X) are adsorbed by means of long chain alkylether fragments only;
- the factor of desorption is in contradiction with the factor of adhesion fixing of molecules (I-IX) on a steel surface [27]. In the case of compounds (XIa-b) the perfluorooxaalkyl fragment possesses weak adsorption on a steel surface. Although at the same time this adsorption force is sufficient for the development of an integral lubricant layer. It contributes to the improvement of antifrictional characteristics;
- the esters (XIa-b) possess sufficient length and iso-structure of the perfluorooxaalkyl fragment. They create more favorable conditions of a boundary friction than the esters with perfluoroalkyl substituents. Thus, the esters (XIa-b) keep the highest amount of lubricants at a steel surface.
|
|
a)
b)
c) |
Fig.
3 Images of steel plates after
boundary friction processes in the presence of the
petroleum-based oil with ester additives: |
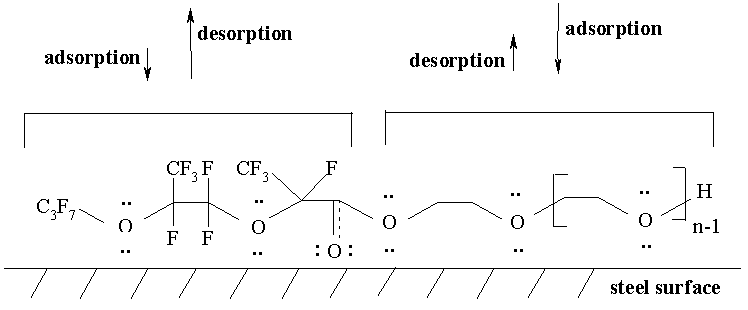
Fig. 4 Arrangement of a monoester (XIa) molecule on a steel surface.
It is known that when PFPE trade names Krytox and Fomblin are used the friction coefficient increases with increasing load [10]. For example, the steady-state the friction coefficient for Krytox is nearly 0.13 with loading 3.5N and 0.17 with loading 8.4N. Our study has been carried out at rather high loadings (294N). The established friction coefficients are comparable to the data of paper [10] which were received at much lower loadings and at using as lubricants instead of additives. Thus, the synthesized ester mixtures (I-XI) are competitive with other types of additives.
Recently the technology of improvement of antiwear properties of lubricants by means of the introduction of nanosized metal powders possessing “mending effect” has been greatly developed [28]. Usually soft metals are used, mostly copper. Here the main difficulty is the organization of stable suspension of the metal powder in oils. To eliminate this problem it is necessary either to modify the metal surface preliminary, for example, by organic compounds [29] or to add to the oil an effective stabilizer of the suspension together with the metal [28].
The synthesized ester mixtures (I-XI) possessing the ability to improve antifrictional characteristics improvement have high suspension power as well. These compounds are excellent stabilizers and create stable dispersions of nanosized Cu powder in petroleum-based oil for a long time (several months). It should be noticed that to create stable dispersions based on petroleum oil, nanosized Cu powder and the mixtures (I-XI) only moderate ultrasonic dispersion is sufficient (for two minutes, operating frequency 22+10% kHz). The data of antifrictional parameters of the Cu suspensions are presented in Table 3.
Table 3 Antifrictional parameters of mixtures with nanosized Cu powder.
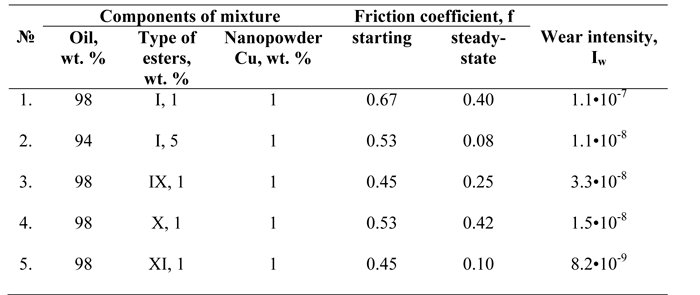
The study of friction coefficient values of the prepared Cu-containing remetallizing mixtures shows that the introduction of the nanosized Cu powder does not influence much the improvement of the friction coefficient. It is caused by the appearance of an unfavorable mixed type of boundary friction of the steel pair. The amount of the stabilizer influences the friction coefficient considerably: increasing the ester’s (I) content five time results in a decrease in the friction coefficient virtually by five times (examples NN 1,2 in Table 3). The presence of the Cu nanoparticles has positive influence on Iw values (examples NN 3,4 in Table 3): this indicator has decreased by one order of magnitude in comparison with examples NN 9,10 in Table 2. In the case of mixture (XI) the Iw has increased more than twice (example N 11 in Table 2).
4 Conclusion
Thus, the synthesized ester mixtures based on perfluorocarbon acid (acid fluoride) and polyols are universal additivies to petroleum-based oils. They simultaneously show positive antifrictional characteristics and stabilizing properties with respect to nanosized Cu powders as well.
The esters with perfluorooxaalkyl substituent (IV, VIII, XI) being used as additives possess the greatest effect of improvement of antifrictional properties of the petroleum-based oils. The presence of polar oxygen atoms in a chain of the fluorinated radical influences affinity of the whole molecule of the additive to a metal surface favorably: adhesion power is increased, the lubricant film on a metal friction surface is sufficient for effective boundary friction.
The additives from the ester mixtures (IX, X) based on PEG-1000 and perfluorocarbon acids (C4F9COOH, C6F13COOH) possess comparably worse wear-resistant properties. The nanosized Cu powders added to the lubricants based on the petroleum-based oil and ester mixtures (I, IX-XI), contribute to the improvement of antiwear properties of the austenitic chromium-containing steel.
Acknowledgments. This research was supported by the Ural Division of Russian Academy of Sciences.
References
- Dai Q., Vurens G. Lubricant Distribution on Hard Disk Surfaces: Effect of Humidity and Terminal Group Reactivity // Langmuir. 1997. Vol.13. N 16. P. 4401-4406.
- Kasai P.H. Perfluoropolyethers: Intramolecular Disproportionation // Macromolecules. 1992. Vol. 25. N 25. P. 6791-6799.
- Fultz G.W., Scott O.S., Chen L.S., Eapen K.C. Stabilization of a linear perfluoropolyalkylether fluid // Tribol. Lett. 1998. Vol. 5. N 4. P. 287-291.
- Zhu J., Liu W., Chu R., Meng X. Tribological properties of linear phosphazene oligomers as lubricants // Tribol. Int. 2007. Vol. 40. P. 10-14.
- Eapen K.C. Additive structural changes during oxidation/corrosion testing of a perfluoropolyalkylether lubricant // Tribol. Lett. 1997. Vol. 3. N 3. P. 283-287.
- Li L., Jones P.M., Hsia Y.-T. Effect of chemical structure and molecular weight on high-temperature stability of some Fomblin Z-type lubricants // Tribol. Lett. 2004. Vol. 16. NN 1-2. P. 21-27.
- John P.J., Lianga J., Cutler J.N. Surface activity of high-temperature perfluoropolyalkylether oil additives // Tribol. Lett. 1998. Vol. 4. NN 3-4. P. 277-285.
- Cutler J.N., Sanders J.H., Fultz G.W., Eapen K.C. The effect of thermal stressing on perfluoropolyalkylethers at elevated temperatures // Tribol. Lett. 1998. Vol. 5. N 4. P. 293-296.
- Trivedia H.K., Sabab C.S., Givan G.D. Thermal stability of a linear perfluoropolyalkylether in a rolling contact fatigue tester // Tribol. Lett. 2002. Vol. 12. N 3. P. 171-182.
- Cheong C.U.A., Stair P.C. In situ studies of the lubricant chemistry and frictional properties of perfluoropolyalkyl ethers at a sliding contact // Tribol. Lett. 2001. Vol. 10. NN 1-2. P. 117-126.
- Kasai P.H., Raman V. Perfluoropolyethers with dialkylamine end groups: ultrastable lubricant for magnetic disk application // Tribol. Lett. 2002. Vol. 12. N 2. P. 117-122.
- Jianbin L., Mingchu Y., Chaohui Z., Guoshun P., Shizhu W. Study on the cyclotriphosphazene film on magnetic head surface // Tribol. Int. 2004. Vol. 37. P. 585-590.
- 13. Veldhuis S.C., Dosbaeva G.K., Benga G. Application of ultra-thin fluorine-content lubricating films to reduce tool/workpiece adhesive interaction during thread-cutting operations // International Journal of Machine Tools and Manufacture. 2007. Vol. 47. P. 521-528.
- 14. Kotov Y.A., Rhee C.K., Beketov I.V., Bagazeyev A.V., Demina T.M., Murzakayev A.M., Samatov O.M., Timoshenkova O.R., Medvedev A.I., Shtols A.K. Production of Copper Nanopowders by Electric Explosion of Wire-Study of Their Oxidation during Storage and Heating in Air // J. Metast. Nanocrystal. Mater. 2003. NN 15-16. P. 343-348.
- Hamida S.B.A., Abdullaha F.Z., Ariyanchiraa S., Mifsudb M., Iborrab S., Corma A. Polyoxyethylene esters of fatty acids: an alternative synthetic route for high selectivity of monoesters // Catal. Today. 2004. Vol. 97. N 4. P. 271–276.
- Tao Z., Bhushan B. Bonding, degradation, and environmental effects on novel perfluoropolyether lubricants // Wear. 2005. Vol. 259. P. 1352-1361.
- Palacio M., Bhushan B. Surface potential and resistance measurements for detecting wear of chemically-bonded and unbonded molecularly-thick perfluoropolyether lubricant films using atomic force microscopy // J. Colloid. Interface Sci. 2007. Vol. 315. P. 261-269.
- 18. Palacio M., Bhushan B. Nanotribological properties of novel lubricants for magnetic tapes // Ultramicroscopy. 2009. Vol. 109. P. 980-090.
- 19. Trivedi H.K., Saba C.S., Carswell L.C., Gschwender L.J., Snyder C.E. Behavior of perfluoropolyalkylethers under simulated dynamic bearing conditions // Tribol. Lett. 1998. Vol. 5. NN 2-3. P. 211-222.
- Trivedia H.K., Forsterb N.H., Saba C.S. Rolling contact fatigue testing of a 3 cSt polyolester lubricant with and without TCP and DODPA/PANA at 177 oC // Tribol. Lett. 2004. Vol. 16. N 3. P. 231-237.
- Padmaja K.V., Rao B.V.S.K., Reddy R.K., Bhaskar P.S., Singh A.K., Prasad R.B.N. 10-Undecenoic acid-based polyol esters as potential lubricant base stocks // Ind. Crops. Prod. 2011. Vol. 35. P. 237-240.
- Lange R.M. Dispersant-viscosity improvers for lubricating oil compositions // Eur. Patent. 1996. N 0 730 022 B1.
- Wan Y., Xue Q., Cao L. Tribological Properties of some Water-Based Lubricants containing Polyethylene Glycol under Boundary Lubrication Conditions // J. Synth. Lubr. 2006. Vol. 13. N 4. P. 375-380.
- Pervova M.G., Kirichenko V.E., Gorbunova T.I., Zapevalov A.Ya., Saloutin V.I. Synthesis and GC-MS study of fluorinated esters derived from trimethylolpropane // Russian J. General Chem. 2008. Vol. 78. N 9. P. 1701-1706.
- Izumisawa S., Jhon M.S. Stability analysis of ultra-thin lubricant films with chain-end functional groups // Tribol. Lett. 2002. Vol. 12. N 1. P. 75-81.
- McGuiggan P.M., Gee M.L., Yoshizawa H., Hirz S.J., Israelachvili J.N. Friction Studies of Polymer Lubricated Surfaces // Macromolecules. 2007. Vol. 40. N 6. P. 2126-2133.
- Lim M.S., Yun Y., Gellman A.J. Interaction of Alcohols and Ethers with a-CFx Films // Langmuir. 2006. Vol. 22. N 3. P. 1086-1092.
- Liu G., Li X, Qin B., Xing D., Guo Y., Fan R. Investigation of the mending effect and mechanism of copper nano-particles on a tribologically stressed surface // Tribol. Lett. 2004. Vol. 17. N 4. P. 961-966.
- Zhou J., Wu Z., Zhang Z., Liua W., Xue Q. Tribological behavior and lubricating mechanism of Cu nanoparticles in oil // Tribol. Lett. 2000. Vol. 8. N 4. P. 213–218.
Recommended for publication by prof. Alexandr Y. Zapevalov
Fluorine Notes, 2012, 80, 5-6

