Received: November, 2011
Fluorine Notes, 2011, 79, 7-8
1-Ferrocenyl-1-Trifluoromethyl-2,2,2-Trifluoroethyl Methacrylate — A New Monomer For Polymer Chemistry
V.I. Dyachenko, L.N. Nikitin, O.A. Mel'nik, S.M. Peregudova, A.S. Peregudov, S.M. Igumnov, A.R. Khokhlov
A.N. Nesmeyanov Institute of Organoelement Compounds Russian Academy of Sciences (INEOS RAS), Russian
Federation, 119991, GSP-1, Moscow, V-334, Vavilova St. 28
e-mail: vic-d.60@mail.ru
Abstract: 1-Ferrocenyl-1-trifluoromethyl-2,2,2-trifluoroethyl methacrylate has been synthesized by the reaction between 1-ferrocenyl-1-trifluoromethyl-2, 2, 2-trifluoroethyl alcohol and methacryloyl chloride in the presence of sodium hydride in anhydrous dimethylformamide (DMF). It has been established its ability to the reversible oxidation-reduction and the radical (co) polymerization in a supercritical carbon dioxide and organic solvents.
Keywords:1-Ferrocenyl-1-trifluoromethyl-2,2,2-trifluoroethyl alcohol, methacryloyl chloride, 1-ferrocenyl-1-trifluoromethyl--2,2,2-trifluoroethyl methacrylate, polymerization.
It is known that the fluorinated polymers, in contrast to the non-fluorinated analogues are more lipophilic and hydrophobic, highly resistant to the oxidation, the action of acids and other aggressive media. Thanks to these valuable properties it has been increasing their expansion in a science of materials, electronics, automotive, fiber-optic industries, everyday usage [1].
However, the modern innovative economic development based on nanotechnologies requires the creation of materials not only having protective properties and structural features, but also the substances carring various functional properties. Among them there are polymers possessing proton or electron conductivity, the materials converting solar energy into electrical energy, the copolymers acting as the sensory systems, the means of a selective drug delivery into the target human cell [2].
Over recent years there has been increasing interest of researchers in polymers based on a ferrocene. It has been mainly due to the fact that the ferrocene, in addition to the structural features of the molecule, has one of the lowest oxidation potential. Moreover, the oxidation of its molecule has not a destructive character and is only related to the change in valence of iron being a part of the ferrocene (Fe2+ to Fe3+). A rezulting ferrocenyl cation under the action of the reducing agent is easily converted back to the original ferrocene changing the color from blue to yellow [3].
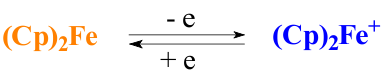
In this context, it has appeared very successfully to use the redox process on the basis of the ferrocene derivatives in the practice of the creating of sensor systems [4,5].
One approach to the creation of biosensors is the inclusion of the redox mediators in the polimer to ensure a contact of the enzyme and the electrode. It is therefore very important to stabilize a mediator in a polymer layer. That is, it should have a group binding it to the polymer matrix. In this regard the ferrocene derivatives of amphiphilic pyrrole type (Ia), which can chemically be attached to the chain by the electrochemical polymerization of pyrrole, have been received [6-8] (see Figure 1). When creating a glucose biosensor the corresponding silicon-containing redox mediators have been attached to the silicate base ( Ib) [9].
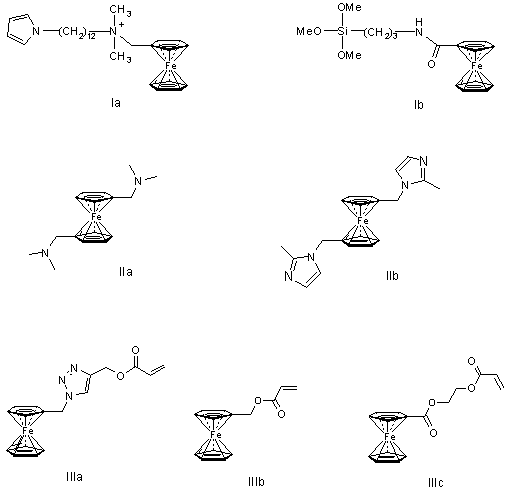
Fig.1.
In order to receive organic polyelectrolytes the nitrogen-containing monomers 1,1'-bis(N,N-dimethylaminomethyl)- and 1,1' -bis[1-(2-methylimidazol-1-yl)methyl] ferrocenes (IIa, IIb) for the copolymerization with 1,4-dibromobutane and α, α-dibromo-p-xylene have been synthesized [10]. To study the electrochemical properties of the ferrocene-containing (meth)acrylates (IIIa-IIIc) and polymers based on them have recently been obtained [11]. Using 1,1-bis(chloroformyl) ferrocene phosphorus-containing liquid-crystalline polymers have been synthesized [12].
The aim of this research is the synthesis of a new fluorine-containing methacrylate having a ferrocenyl group as a redox mediator (see Scheme 1) and the study of its physico-chemical properties.
Initial 1-ferrocenyl-1-trifluoromethyl-2,2,2-trifluoroethanol (1) [13] was prepared from commercially available hexafluoroacetone and ferrocene according to a previously described method [14]. The corresponding 1-ferrocenyl-1-trifluoromethyl-2,2,2-trifluoroethylmethacrilate (3) was obtained by the reaction between alcohol (1) and methacryloyl chloride (2).
Scheme 1.
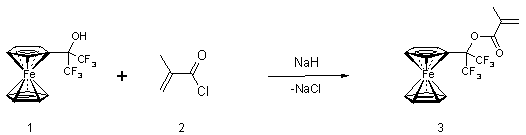
The reactions 1 and 2 can be easily carried out in anhydrous DMF in the presence of sodium hydride forming 3in good yield.
Methacrylate 3 is a red-orange crystalline substance, soluble in almost all organic solvents and stable in storage. It is easy (co)polymerized in a solution in the presence of an initiator of the radical polimerization - azoisobutyronitrile at 60-70°C.
Using the method of the cyclic voltammetry (CV) the electrochemical properties of methacrylate 3 have been studied (see Fig. 2). It is shown that the oxidation is carried out in one electron reversible stage. Oxidation potential (E1/2 = +0.63 V) is shifted to the positive region of potentials in comparison with the ferrocene (E1/2 = +0.40 V) at 230 mV, indicating the electron acceptor character of a fluorocontaining substituent.
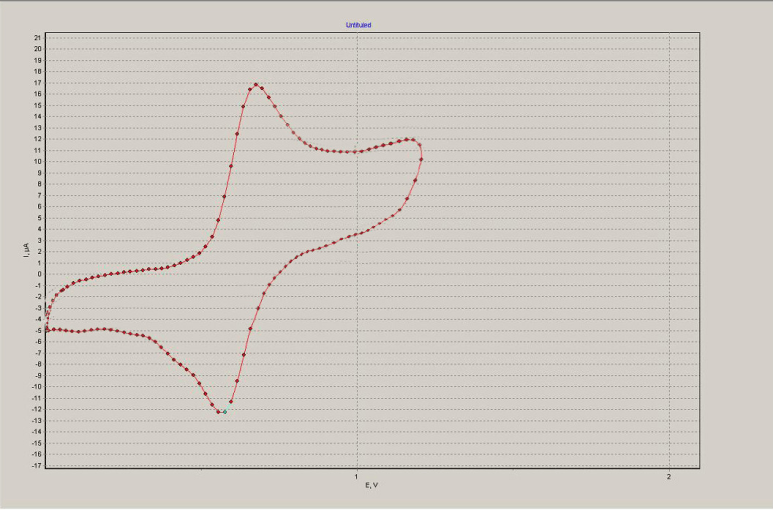
Fig. 2. Cyclic voltammogram of methacrylate 3 (a solvent – acetonitrile, a background electrolyte - 0.1 M Bu4NPF6, a working electrode - glassy carbon, an auxiliary electrode - Pt, a reference electrode - saturated calomel electrode (SCE)).
Experimental
1H NMR spectra were recorded in CDCl3 on the instrument Bruker Avance (TM) 600 (600.22 MHz), the NMR spectrum of 19F - on the instrument Bruker Avance (TM) 300 (282,40 MHz). As the internal standard Me4Si (1H and 13C) was used, as the external standard - SF3CO2H (19F). The mass spectrum was recorded on a quadrupole mass spectrometer FINNIGAN MAT INCOS 50. Direct input, the ionization energy of 70 eV.
1-Ferrocenyl-1-trifluoromethyl-2,2,2-trifluoroethyl methacrylate 3. 0.24 g (10 mmol) of 60% sodium hydride in liquid petrolatum was added by portions to a solution of 3.52 g (10 mmol) of alcohol 1 in 25 ml of anhydrous DMF in a glass flask at 20°C with mixing by a magnetic stirrer. At the end of the release of hydrogen 10 mg of butylated hydroxytoluene and 1.14 g (11 mmol) of methacryloyl chloride drop by drop were added in the reaction mixture , while maintaining the temperature of 20°C. After the addition of 2 the contents the reaction mixture was being stirred for 1 hour and poured into150 ml of cold water. The reaction product was extracted with petroleum ether (50 ml x 2), the solution was dried with anhydrous sodium sulfate and evaporated in vacuum. The reaction product was isolated by column chromatography, eluent - hexane. 2.9 g of red-orange oil crystallized upon cooling was obtained. Yield 69%. Melting point 58-59°C (petroleum ether). 1H NMR spectrum (δ, ppm): 1,95 (s, 3H, CH3); 4,24 (w.s., 5H, C5H5); 4,37 (w.s,, 2H, 2CH, (C5H4), 4,46 (w.s., 2H, 2CH, (C5H4), 5,71 (s, 1H, CH2), 6,20 (s, 1H, CH2). 19F NMR spectrum (δ, ppm.): 8,64 s. Mass spectrum, m/z: [M]+ (420). Found (%): C, 48.74: H, 3,40; F, 26,90. C17H14F6FeO2. Calculated (%): C, 48,60; H, 3,36; F, 27,13.
Electrochemical studies were carried out in an atmosphere of dry argon in a 0.1 M solution of Bu4NPF6 in acetonitrile. Measurements were carried out in a three-electrode electrochemical cell with undivided cathodic and anodic space. The potentials were measured relative to an aqueous saturated calomel electrode (SCE). An auxiliary electrode was a platinum plate, which is located in the cell. The working electrode was the front part of the glassy carbon rod (the area of the disc was 2 mm2). Cyclic voltammograms were recorded using a potentiostat IPC-Pro M. The rate of potential scan was 200 mV/sec. The concentration of a test compound was 10-3 M.
Thus, 1-ferrocenyl-1-trifluoromethyl-2,2,2-trifluoroethyl methacrylate - a new monomer to polymer chemistry was sinthesized. Its ability to (co)polymerization in the conditions of radical initiation with the formation of "side-chain" ferrocene-containing polymers was shown. Its physicochemical properties were studied. Its reversible one-electron oxidation-reduction was established which confirms the possibility of its use as an antioxidant additive in polymers capable of attaching to the polymer chain. In the future these features of 3 can also be used to create biosensor test systems.
This work was financially supported by the Presidium of RAS P7 Program and by the grant of RFFI # 11-03-12068–ofi-m,
2011.
References
- Fluorine compounds. Synthesis and Application, ed. N. Ishikawa, translation from Jap., Mir, 1990.
- The program of the International Forum on Nanotechnology «Rusnanotech 2011", Moscow, 2011.
- A. N. Nesmeyanov. Ferrocene and related compounds. Moscow: Nauka, 1982.
- R.Deschenaux, Polym. Mater. Sci. Eng., 2002, 86, 97.
- F.Turpin, D.Guillon, R.Deschenaux, Mol. Cryst. Liq., Cryst. Sci. Technol. Sect. A, 2001, 362, 171-175.
- S.Cosnier, Biosens. Bioelectron., 1999, 14, 443-456.
- S.Cosnier, C.Innocent, Y.Jouanneau, Anal. Chem., 1994, 66, 3198-3201.
- (a) S.Cosnier, L.Allien, L.Coche-Guérente, C.Innocent, P.Labbé, P.Mailley, Sens. Mater., 1996, 8, 169-177; (b) S.Cosnier, B.Galland, C.Innocent, J. Electroanal. Chem., 1997, 433, 113-119.
- (a) J.Gun, O.Lev, Anal. Chim. Acta, 1996, 336, 95-106; (b) J.Gun, O.Lev, Anal. Lett., 1996, 29, 1933-1938].
- Ye Gao and Jean'ne M.Shreeve, Journal of Polymer Science, Part A: Polymer Chemistry, 2005, 43, 5(1), 974–983.
- C.G.Hardy, L.Ren, T.C.Tamboue, C.Tang, Journal of Polymer Science, Part A: Polymer Chemistry, 2011, Vol. 49, 1409–1420.
- S.Senthil, P.Kannan, Journal of Polymer Science, Part A: Polymer Chemistry, 2001, 39, 14, 2396-2403.
- M.I.Bruce, F.G.Stone, B.I.Thomson, J. Organomet. Chem., 1974, 77, 1, 77.
- V.I.Dyachenko, A.F. Kolomiets, A.V. Fokin, Abstracts of the Fifth All-Union Conference on Organometallic
Chemistry, Riga, 1991.
Recommended for publication by Prof. Sergei R. Sterlin
Fluorine Notes, 2011, 79, 7-8
