Received: September, 2011
Fluorine Notes, 2011, 79, 5-6
IMPROVEMENT OF THE METHOD FOR SYNTHESIS OF PERFLUOROETHYLISOPROPYLKETONE
I.M. Fenichev, Y.I. Babenko, T.A. Bispen, A.S. Vasil'ev, D.D. Moldavsky
FCUE Russian Scientific Center "Applied Chemistry", 197198, Russia, St. Petersburg, Dobrolubov av. 14
e-mail: vbarabanov@rscac.spb.ru
Abstract: The results of investigations regarding new methods of synthesis of perfluoroisopropylketone using an aprotic polar solvent have been represented. The mechanism of synthesis of perfluoroethylisopropylketone has been established. The influence of water in a solvent on the product"s yield has been shown. Purification methods of perfluoroethylisopropylketone from 2-perfluoromethylpentene and perfluoro-2-methyl-3- oxahexanoylfluoride have been developed
Keywords:perfluoroisopropylketone, fire extinguishant, a polar solvent, perfluoroalcoholate, 2-perfluoromethylpentene, perfluoro-2-methyl-3-oxahexanoylfluoride.
Recently it has been observed an increased interest in perfluoroisopropylketone (hereafter ketone) due to its fire extinguishing properties [1]. In addition, this ketone is a valuable intermediate product for the synthesis of a variety of fluoroorganic compounds [2]. The primary method for synthesis of the ketone described in several papers [3-5], consists in the reaction of hexafluoropropene (hereafter HFP) with fluoroanhydride of perfluoropropionyl acid (hereafter FAPA) catalyzed by alkali metal fluorides in the medium of aprotic polar solvents such as acetonitrile or diglyme:
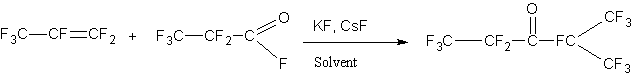
The process is carried out in an autoclave at temperatures 200C-85°C with CsF and KF as a catalysts . The main problem of this synthesis is the deleivery of the basic substanses. FAPA in is not obtained in our country, but it is relatively easily synthesized by isomerization of hexafluoropropene oxide (HFPO) [6-8]:
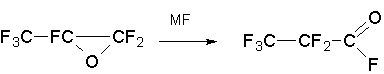
Alkali metal fluorides KF or CsF have been used as catalysts in this process just in the synthesis of the ketone.
We studied this reaction and found that the isomerization proceeds at high rate under the same conditions as the synthesys of the ketone, namely, at 20-85°C, depending on the catalyst. Thus, initially, the process of the ketone synthesis comprises the stage of isomerization of the HFPO to FAPA , and then the stage of the reaction between FAPA and HFP. Studying the process it has been seemed appropriate to attempt to combine the isomerization of HFPO and the synthesis of the ketone into one stage. Indeed, it has been possible and we found no difference in the use of HFPO and FAPA. The mechanism of reaction as in the case FAPA, and in the case of HFPO represents the formation of perfluoropropylate which then reacts with the HFP:
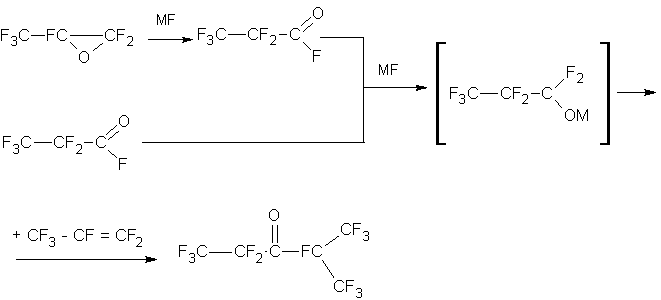
In both cases the rate of formation of perfluoropropylate is much higher than the rate of a reaction of alcoholate with HFP.
Having studied the synthesis of this ketone, we found that the yield of the ketone depended on the water content in the solvent. Table 1 shows the dependence of the yield of ketone from the water content in acetonitrile (water content in CsF, KF ≤ 0,001% mass).
Table 1.
| Water content, % mass |
0,01 |
0,02 |
0,04 |
0,06 |
0,09 |
0,10 |
0,12 |
| The yield of the ketone, % |
63,5 |
61,5 |
61,0 |
53,0 |
39,0 |
30,0 |
22,0 |
The reduction of the yield could be apparently caused by the formation of complexes MF•H2O,
having non-catalytic activity.
The process of the ketone synthesis is accompanied by the formation
of by-products, mainly 2-perfluoromethylpenthene(dimer HFP) and perfluoro-2-methyl-3-oxahexanoylfluoride
(dimer HFPO). Their total content may reach 30%. Figures 1 and 2 show the chromatograms of the ketone.
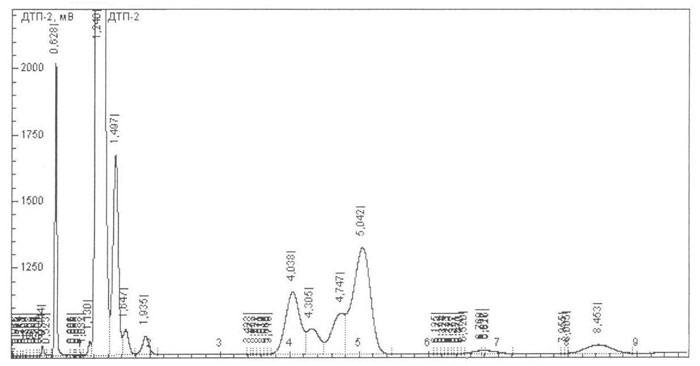
Fig.1. Chromatogram of the crude ketone on the silochrom column.
Table 2.The results of chromatographic analysis on the silochrom column.
|
Time, min |
Compound |
Area |
Peak height |
Area, % |
|
|
0.017 |
3.704 |
5.187 |
0.005 |
||
|
0.066 |
13.343 |
5.107 |
0.017 |
||
|
0.114 |
12.586 |
4.899 |
0.016 |
||
|
0.161 |
12.303 |
4.766 |
0.015 |
||
|
0.209 |
11.865 |
4.631 |
0.015 |
||
|
0.258 |
11.387 |
4.366 |
0.014 |
||
|
0.306 |
10.943 |
4.157 |
0.014 |
||
|
0.354 |
10.399 |
4.061 |
0.013 |
||
|
0401 |
9.699 |
3.764 |
0.012 |
||
|
0.444 |
90.834 |
37.535 |
0.112 |
||
|
0.523 |
18.630 |
6.026 |
0.023 |
||
|
0.628 |
2336.168 |
1097.099 |
2.891 |
||
|
0.886 |
6.483 |
2.713 |
0.008 |
||
|
0.895 |
3.955 |
2.113 |
0.005 |
||
|
0.935 |
1.854 |
2.429 |
0.002 |
||
|
0.983 |
4.900 |
2.076 |
0.006 |
||
|
1.032 |
4.468 |
2.012 |
0.006 |
||
|
1.130 |
158.219 |
53.430 |
0.196 |
||
|
1.240 |
58074.430 |
10423.621 |
71.855 |
||
|
1.497 |
4104 289 |
751.302 |
5.078 |
||
|
1.647 |
530.221 |
92.690 |
0.656 |
||
|
1.935 |
453.857 |
67.799 |
0.562 |
||
|
3.423 |
1.121 |
1.008 |
0.001 |
||
|
3.472 |
1.724 |
1.280 |
0.002 |
||
|
3.522 |
2.719 |
1.717 |
0.003 |
||
|
3.571 |
3.843 |
2.045 |
0.005 |
||
|
3.619 |
5.056 |
2.464 |
0.006 |
||
|
3.668 |
5 626 |
2.498 |
0.007 |
||
|
3.718 |
5.648 |
2.532 |
0.007 |
||
|
4.038 |
3445.575 |
233.143 |
4.263 |
||
|
4.305 |
1207.583 |
95.006 |
1.494 |
||
|
4.747 |
1984.256 |
151.962 |
2.455 |
||
|
5.042 |
7093.609 |
398.766 |
8.777 |
||
|
6.125 |
1.167 |
1.031 |
0.001 |
||
|
6.174 |
1.731 |
1.214 |
0.002 |
||
|
6.224 |
2.443 |
1.471 |
0.003 |
||
|
6.273 |
3.149 |
1.636 |
0.004 |
||
|
6.323 |
3.510 |
1.782 |
0.004 |
||
|
6.372 |
3.559 |
1.856 |
0.004 |
||
|
6.421 |
3.757 |
1.838 |
0.005 |
||
|
6.470 |
4.706 |
2 480 |
0.006 |
||
|
6.520 |
6.956 |
3.490 |
0.009 |
||
|
6.768 |
113.036 |
12.555 |
0.140 |
||
|
6.817 |
36.307 |
12.796 |
0.045 |
||
|
6.826 |
134 494 |
12.161 |
0.166 |
||
|
7.955 |
1.347 |
1.277 |
0.002 |
||
|
8.005 |
3.019 |
1.963 |
0.004 |
||
|
8.453 |
860.753 |
33.186 |
1.065 |
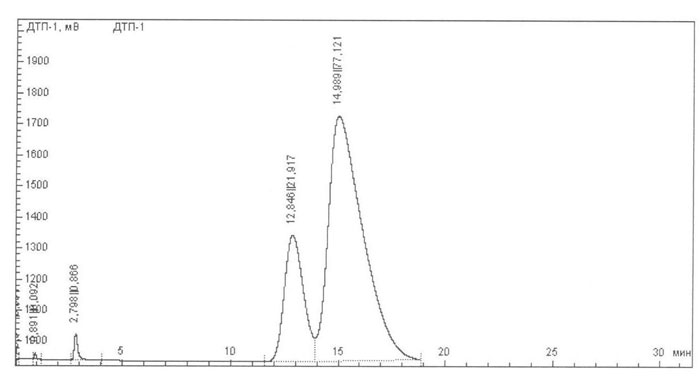
Fig. 2. Chromatogram of the crude ketone on the Porapak-Q column.
Table 2.The results of chromatographic analysis on the Porapak-Q column.
|
# |
Time, min |
Compound |
Area |
Peak height |
Area, % |
|
1 |
0.011 |
4.207 |
1.572 |
0.004 |
|
|
2 |
0.891 |
105.621 |
21.080 |
0.092 |
|
|
3 |
2.798 |
994.668 |
83.407 |
0.866 |
|
|
4 |
12.846 |
25182.964 |
406.141 |
21.917 |
|
|
5 |
14.989 |
88611.841 |
790.553 |
77.121 |
The seperation of the ketone by rectification is inefficient since the boiling points of the ketone, HFP dimer and HFPO dimer are 48,5°C, 46-51°C and 56°C, respectively.
HFPO dimer (Fig. 1, retention time is 1.497min) is easily separated from the reaction mixture by the aqueous alkali treatment :
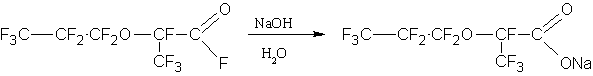
This process is accompanied by ketone hydrolysis:
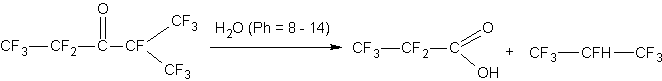
Figure 3 shows the dependence of hydrolysis of the ketone on the pH of an aqueous solution.
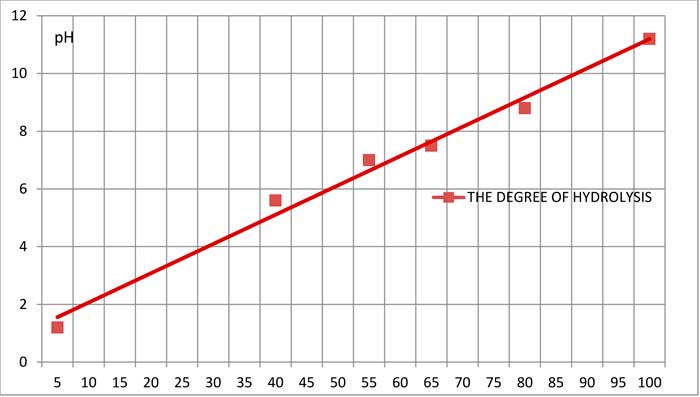
Fig. 3. Hydrolysis of the ketone in aqueous solutions (temperature is 15°C, mixing rate is 350 r / min, the volume ratio of ketone - solution is 1:25)
To separate the HFP dimer (Fig. 1, retention time is equal to 12.846) from the ketone the reactions of this ketone with several compounds, in particular with chlorofluoride and hydrogen were examined:
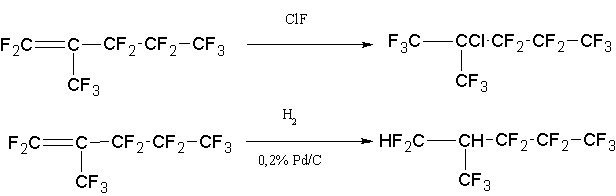
Both of these reactions proceed almost quantitatively. Synthesized compounds have boiling points
81 and 70 °C, respectively, and are easily separated by rectification. However, due to the high activity
of ClF, the preference was given to hydrogenation. Under carried investigations it have been created
an experimental laboratory plant with productivity up to 5.5 kg / day.
The experimental part
The solvent, dried to a residual water content of not more than 0,008% by weight and 85 g of dried and powdered CsF are loaded into the steel reactor (12X18H10T) shown in Figure 2, equipped with a Goffer valve, magnetic stirrer and a pressure gauge. Under the constant heating and stirring the mixture of HFPO and HFP in a 1:1 ratio of up to 4.5 kg is added into the reactor. The temperature of the reactor supports about 20-25 °C. The reaction is controlled by the drop in pressure. After carrying out the process (after 4 - 5 hours), the mixture is cooled, separated from the solvent and the catalyst. The content of the pure ketone in the mixture is 61.5%.
The catalyst recycling reduces the rate of interaction between HFPO and HFP in 1.5 - 2 times and decreases the yield to 35%.
Purification of ketone from the HFPO dimer is carried out in the autoclave reactor. Synthesized crude
ketone and alkaline solution at a ratio of 1:10 are loaded into the reactor. The process is carried
out at room temperature for 3 hours. The resulting pressure is throttled. The reaction is controlled
by the drop in pressure.
Ketone purified from the HFPO dimer is separated by a separating
funnel, weighted and analyzed and then purified from the HFP dimer.
The activated catalyst Pd 0,2% / C (0,16l) and perfluorinated liquid containing HFP dimer are loaded into a temperature-controlled steel autoclave reactor with a capacity 0.8 liters. The reactor is sealed and H2 is passed at 0-20°C. The duration of the process depends on the pressure, at pressures 0,35-1,5 MPa process takes 0.5-1 h, respectively. The reaction is controlled by the drop in pressure. At the end of the process the gas excess are removed, the product is weighted and analysized.
References
- Preparation of compounds with perfluoroalkyl groups. Pat. Germany 1973864 (2009).
- Furin G.G. In the development of new synthetic methods for fluorine-containing heterocyclic compounds / / J. Org. Chem. - 1994. - V. 30, 11, 1704-1758.
- Pat. U.S. 3185734 (1965).
- Pat. U.S. 4136121 (1979).
- Kolenko I.P.; Plashkin V.S.; Ovchinnikov G.F. J. Vser. Chem. Society. Mendeleev, 1974,19, 707.
- Application # 58-35978 (Japan) / Yonotasi A.; Yoshio A.
- Pat. # 58-38231 (Japan) / Yonosuke I.; Yoshio A.; Hiroyuki S.
- Pat. U.S. number 5684193, 1997.
Recommended for publication by V. Kornilov
Fluorine Notes, 2011, 79, 5-6
