Fluorine Notes, 2011, 78, 1-2
UNCONVENTIONAL REACTIONS OF TRIMETHYL(TRIFLUOROMETHYL)SILANE
A.D. Dilman
N. D. Zelinsky Institute of Organic Chemistry, 119991 Moscow, Leninsky Prospect 47, Russia
E-mail:
dilman@ioc.ac.ru
Abstract: Reactions of trimethyl(trifluoromethyl)silane which do not result in nucleophilic transfer of CF3-group are discussed.
Keywords: trimethyl(trifluoromethyl)silane, difluorocarbene.
Trimethyl(trifluoromethyl)silane (Me3SiCF3, the Ruppert-Prakash reagent) has found widespread applications for nucleophilic trifluoromethylation reactions [1]. Indeed, Me3SiCF3 serves as a source of CF3-carbanion towards wide variety of electrophilic fragments such as C=X bonds, heteroatom-centered electrophiles, and transition metal complexes. The general feature of all these processes is that they proceed in the presence of an appropriate Lewis basic activator (typically fluoride), which interacts with the silicon atom generating highly reactive five-coordinate species. Herein we highlight reactions of Me3SiCF3 of other types, which likely proceed by different mechanisms or lead to products different from products of nucleophilic trifluoromethylation.
Considering reactivity of Me3SiCF3, two sites of its molecule can be attacked by nucleophilic and electrophilic reagents. Nucleophiles (or Lewis bases) readily approach the silicon atom that induces fission of Si-C bond (path a). The probable site for approach of electrophiles (or Lewis acids) is the fluorine atom and this pathway is expected to lead to the cleavage of strong C-F bond (path b). Both pathways, in principle, can give the same difluorocarbene species.
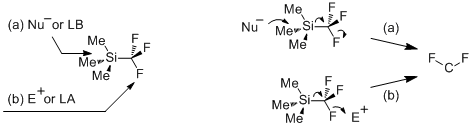
While Me3SiCF3 is relatively stable to the action of protic acids, it readily reacts with triflic acid under solvent-free conditions to give difluoromethyltriflate 1 along with fluorotrimethylsilane [2]. It was suggested that in this reaction the proton abstracts the fluoride from CF3-group accompanied by transfer of the Me3Si-group to the triflate fragment generating the difluorocarbene. The latter is rapidly trapped by triflic acid to give the product 1. At the same time, HF and silyltriflate, which were formed at the step of carbene generation, provide fluorotrimethylsilane. The reaction of Me3SiCF3 with TfOH is accelerated by Lewis acids, and best results were achieved using catalytic amount (1 mol %) of titanium tetrachloride. The Lewis acid may either increase acidity of TfOH, or accelerate equilibrium interaction of HF and silyltriflate.

The reaction of Me3SiCF3 with boron triflate leads to the formation of silyl triflate [2]. It is likely that in this case, similar to reaction with TfOH, the fission of C-F and C-Si bonds occurs in a concerted manner with extrusion of difluorocarbene.

Sodium borohydride reacts with Me3SiCF3 in diglyme at room temperature affording (difluoromethyl)trimethylsilane 2 in good yield [3]. Of special note is that at least three from four hydrides are effectively used thereby allowing to employ substoichiometric amount (0.33 equiv.) of NaBH4. This procedure allows straightforward to access to silane 2, which is more difficult to synthesize by other methods. Concerning reaction mechanism, it was proposed that Me3SiCF3 is activated by borohydride anion that leads to the elongation of Si-CF3 bond and increase of negative charge on CF3-group that, in turn, elongates C-F bond. Subsequent substitution of fluorine by hydride and regeneration of BH4-anion completes the formation of silane 2.

It was recently demonstrated that Me3SiCF3 can be used as efficient source of difluorocarbene [4]. Thus, treatment of alkenes and Me3SiCF3 with catalytic amount of tetrabutylammonium difluorotriphenylsiliconate in tetrahydrofuran results in the formation of difluorocyclopropanes. The reaction proceeds under mild conditions but efficiently works only for electron rich alkenes — this is a typical phenomenon for reactions of difluorocyclopropane.
In this process, the fluoride ion attacks the silicon to give five-coordinate complex, which generates difluorocarbene either by synchronous decomposition (as shown) or through the intermediate formation of free trifluoromethyl carbanion. At the final step, the difluorocarbene is trapped by the olefin.
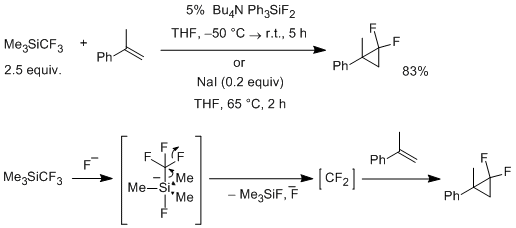
In alternative procedure, Me3SiCF3 is activated by sodium iodide (20 mol %) at elevated temperature (65°C). This protocol has wider scope compared to fluoride-catalyzed procedure, and it was applied to difluorocyclopropanation of various styrenes. Though authors did not suggest the mechanistic scheme, it can proposed that attractive interaction between the iodide ion and silicon inducing polarization of Si-CF3 bond may play important role in this process.
The system Me3SiCF3/NaI was used for the cyclopropantion of alkynes [4]. The reaction is performed in a sealed tube at 110 °C and affords difluorocyclopropenes in high yields.

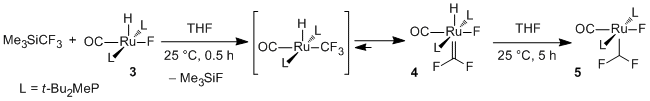
The reaction of Me3SiCF3 with complexes of transition metals in the presence of fluoride or alkoxide anions is frequently employed to generate stable species with the CF3-group attached to metal such as Cu, Ni or Pd [5]. However, when similar transfer of the CF3-group was applied to Ru, Os and Rh complexes, the formation of difluorocarbene complexes was observed [6]. For example, reaction of Me3SiCF3 with ruthenium complex 3 occurred rapidly to give compound 4, which could be isolated in crystalline state. It was suggested that 4 is produced from initially generated Ru-CF3 intermediate. Though compound 4 is stable in non-polar solvents, in tetrahydrofuran it slowly undergoes rearrangement into difluoromethyl-susbtituted complex 5. The overall transformation of 3 to 5 corresponds to the insertion of difluorocarbene into Ru-H bond.
Sulfur trioxide interacts with Me3SiCF3 to give the product of insertion of SO3 between the silicon and the CF3-group [7]. The reaction was carried out at –196 to 25°C in Freon 113 (1,1,2-trichloro-1,2,2-trifluoroethane). The authors did not propose the mechanism of this intriguing reaction. Nevertheless, it seems likely that extremely high electrophilic character of the sulfur trioxide is the key factor responsible for this transformation. Direct attack of sulfur at the CF3-group with simultaneous transfer of the Me3Si-group to the oxygen atom through four-membered transition state cannot be excluded.
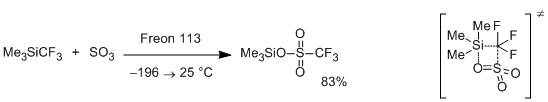
As follows from the reactions outlined above, Me3SiCF3 has diverse reactivity extending beyond conventional nucleophilic trifluoromethylation. In all processes discussed herein either the CF3-group or Si-CF3 bond is involved. It would be a new direction in the chemistry of Me3SiCF3 if some reactions not affecting Si-CF3 fragment are discovered.
Acknowledgement. We are grateful to the Ministry of Science (project MD-1151.2011.3), the Russian Academy of Sciences (program # 7), and the Federal program "Scientific and educational personnel of innovative Russia".
References
1. (a) Prakash, G. K. S.; Yudin, A. K. Chem. Rev. 1997, 97, 757–786. (b) Singh, R. P.;
Shreeve, J. n. M. Tetrahedron 2000, 56, 7613–7632. (c) Prakash, G. K. S.; Mandal,
M. J. Fluorine Chem. 2001, 112, 123–131. (d) Dilman, A. D.; Levin, V. V. Eur. J.
Org. Chem. 2011, 831–841.
2. Levin, V. V.; Dilman, A. D.; Belyakov, P. A.; Struchkova,
M. I.; Tartakovsky, V. A. J. Fluorine Chem. 2009, 130, 667–670.
3. Tyutyunov,
A. A.; Boyko, V. E.; Igoumnov, S. M. Fluorine Notes 2011, 74, 1; /public/2011/1_2011/letters/letter2.html.
4. Wang, F.; Luo, T.; Hu, J.; Wang, Y.; Krishnan, H. S.; Jog, P. V.; Ganesh, S. K.; Prakash, G. K.
S.; Olah, G. A. Angew. Chem. Int. Ed. 2011, 50, 7153–7157.
5. (a) Dubinina,
G. G.; Furutachi, H.; Vicic, D. A. J. Am. Chem. Soc. 2008, 130, 8600-8601. (b) Dubinina,
G. G.; Brennessel, W. W.; Miller, J. L.; Vicic, D. A. Organometallics 2008, 27,
3933-3938. (c) Morimoto, H.; Tsubogo, T.; Litvinas, N. D.; Hartwig, J. F. Angew. Chem. Int. Ed. 2011,
50, 3793–3798. (d) Naumann, D.; Kirij, N., V.; Maggiarosa, N.; Tyrra, W.; Yagupolskii, Y., L.; Wickleder,
M., S. Z. Anorg. Allg. Chem. 2004, 630, 746–751.
6. (a) Huang, D.; Caulton,
K. G. J. Am. Chem. Soc. 1997, 119, 3185-3186. (b) Huang, D.; Koren, P. R.; Folting,
K.; Davidson, E. R.; Caulton, K. G. J. Am. Chem. Soc. 2000, 122, 8916–8931. (с)
Goodman, J.; Grushin, V. V.; Larichev, R. B.; Macgregor, S. A.; Marshall, W. J.; Roe, D. C. J. Am.
Chem. Soc. 2009, 131, 4236-4238.
7. Holfter, H.; Kirchmeier, R. L.; Shreeve,
J. M. Inorg. Chem. 1994, 33, 6369–6372.
Recommended for publication by Prof. S. M. Igoumnov
Fluorine Notes, 2011, 78, 1-2
