Received: July, 2011
Fluorine Notes, 2011, 78, 3-4
The Influence Of Substituents In Allyl Alcohol On The Reaction With Polyfluoroalkylchlorosulphites
Rakhimov A.I1, Nikishin A.I2, Miroshnichenko A.V.1, Do Duong Phuong Thao1
1Volgograd State Technical University, Russia, 400131, Volgograd, Lenin Prosp., 28,
e-mail:
organic@vstu.ru
2N. D. Zelinsky Institute of
Organic Chemistry, 119991 Moscow, Leninsky Prospect 47, Russia
e-mail: nika@ioc.ac.ru
Abstract: It is shown, that at obtaining ether the introduction of phenyl substituent or chlorine atoms into allyl alcohol influences its reaction with polyfluoroalkylchlorosulphites: the reaction of phenyl-substituted (cinnamic) alcohol goes in the presence of potassium carbonate (analogously to allyl alcohol), and trichloroallyl alcohol needs catalysis with N, N-dimethylformamide.
Keywords:Allyl alcohol, trichloroallyl alcohol, cinnamic alcohol, allyl ethers, polyfluorinated alcohol, N,N-dimethylformamide, polyfluoroalkylchlorosulphite.
It is known, that saturated monoatomic and aliphatic-aromatic alcohols react with polyfluoroalkylchlorosulphites (PFACS) over catalyst N,N-dimethylformamide (DMF) forming ethers [1-6]. However, in case of the reaction of polyfluoroalkylchlorosulphite with cinnamic alcohol (catalysis of DMF) one can observe the formation of polyfluorinated alcohol and cinnamyl chloride (the reaction goes similarly to allyl alcohol [7,8]). The chlorosulphite hydrolysis may pass according to the scheme as follows:
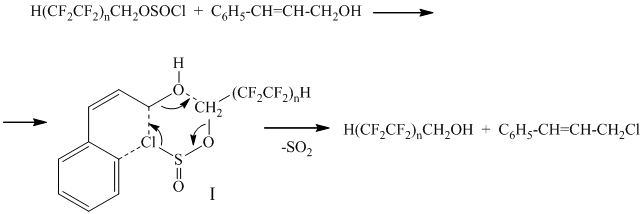
n=2,3;
Chlorine in chlorosulphite group attacks carbon of methylene group in cinnamic alcohol with simulteneous nucleophilic substitution of chlorosulphite group for hydroxyl one through the transition state, which decomposition is accompanied by isolation of sulphur dioxide.
Cinnamic ether of polyfluorinated alcohol is formed as by-product, at that when using the longer chain chlorosulphite (n=3) the ether yield rises up to 20%. This may be due to the increasing of steric factor with polyfluoroalkyl substituent volume going up, which prevents the formation of transition state leading to polyfluorinated alcohol.
Besides that, an oligomer which is a brown viscous glass-like mass was isolated out of reaction mass. The oligomer is water-insoluble, at heating up it dissolves in ethanol and diethyl ether. It is obvious, that 1-phenyl-3-chloropropene-1 formed under the reaction conditions is easily oligomerized.
The application of potassium carbonate which promotes the orientation of reaction centers of interacting molecules of PFACS and cinnamic alcohol allows changing of reaction direction:

The ethers of 32 -43% yield (Table 1) have been obtained as a result of the reaction of polyfluoroalkylchlorosulphites with cinnamic alcohol in the presence of potassium carbonate.
Table 1. The Yield Of Cinnamic Alcohol Polyfluorinated Ethers.
|
# |
PFACS |
Basic Reagent |
Ether Yield, % |
|
1 |
n = 3 |
DMF |
20.0 |
|
2 |
n = 2 |
K2CO3 |
42.7 |
|
3 |
n = 3 |
K2CO3 |
32.5 |
Reaction of polyfluoroalkylchlorosulphites with cinnamic alcohol had been carried in two stages.
During first stage reagents (cinnamic alcohol, potassium carbonate and polyfluoroalkylchlorosulphite) were mixed in chloroform at –10÷–5 °C, while there was the appearance of color–the solution became orange-pink. Further, at second stage the reaction mass was heated up to 30-40 °C and kept for 6 hours. At that, the coloring disappeared. Then the residue of potassium chloride and potassium bicarbonate had been filtered, the solvent had been deleted, and the residue had been distilled in vacuum.
The appearance of coloring at first stage is probably connected with forming of stable at low temperatures complex polyfluoroalkylchlorosulphites – cinnamic alcohol – potassium carbonate. UV-spectra of colored solution points to that: a peak is shown in a visible area at 490 nm, which disappears at heating up together with solution discoloration. The ethers obtained are clear high-boiling liquids which become a viscous and red-brown upon standing, the typical for quinones absorption bands appear in the IR- and UV-spectra [9].
The reaction of polyfluoroalkylchlorosulphite (1,1,5-trihydroperfluoropentylchlorosulphite was studied) with trichloroallyl alcohol in the presence of potassium carbonate under conditions identical for cinnamic alcohol goes with low yield of ether (yield is 9,2%). In that case a complex mixture of dehydrochlorination and hydrolysis products is formed due to interaction of potassium carbonate and trichloroallyl alcohol which has a high acidity of the presence of chlorine.
In connection with that the catalysis of DMF has been used as in the case of reaction of saturated monoatomic and aliphatic-aromatic alcohols with polyfluoroalkylchlorosulphites [1-5]:
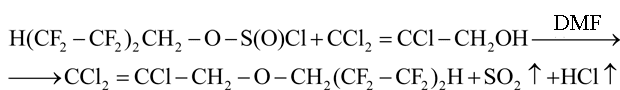
Trichloroallyl alcohol was introduced into reaction in the form of associates with DMF (ratio 1:0.05 mole) in chloroform solution. Associates are formed due to hydrogen bonds between O(2) atom in DMF molecule and mobile hydrogen atoms of H(1) and H(2) in the alcohol molecule:
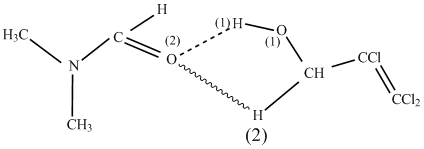
The polarization of H(1)-O(1) bond (bond becomes longer) goes inside associates and proton becomes more mobile.
The reaction was carried out in two stages. At first stage PFACS was dosed into solution obtained at –10°C. Most probably, as it is shown in work [6], in this case a forming of six-membered polar complex is taking place, in which atoms of O(1)-C(3) and H(1)-Cl are included. High nucleophility of chlorine atom and large positively charged proton of alcohol HO-group favors the formation of such complex:
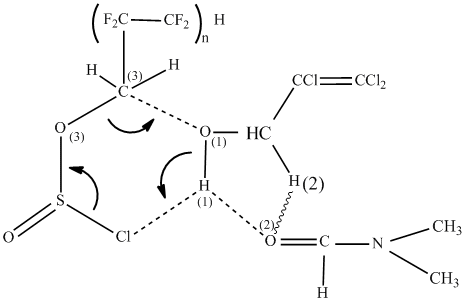
At second stage of decomposition of complex was carried out by heating up of the reaction mixture up to 25-30 and S-Cl bonds with isolating of SO2 and HCl molecules and regeneration of DMF molecule and formation of ether molecule:
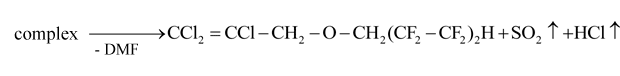
Upon distillation of solvent (chloroform) the product had been distilled. The yield of trichloroallyl ether of 1,1,5-trihydroperfluoropentanol was 53.0% (Table 2).
Table 2. Yield of 1,1,5- trihydroperfluoropentanole ethers.
|
# |
Alcohol and main reagent |
Ether's Yield, % |
|
1 |
Allyl Alcohol, K2CO3 |
40.4 |
|
2 |
Allyl Alcohol, DMF |
traces |
|
3 |
Trichloroallyl alcohol, K2CO3 |
traces |
|
4 |
Trichloroallyl alcohol, DMF |
53.0 |
IR- and 1H NMR- spectroscopy methods were used to study the structure of obtained unsaturated ethers of polyfluorinated alcohols.
A strong narrow band at 1150 - 1060 cm–1 is typical for C-O-C bonds in IR-spectra of polyfluorinated ethers, which is caused by asymmetric stretching vibrations. In the field of 1250 – 1050 cm –1 C–F absorption also appears in the form of a strong broad band, whose width is different from the absorption band of the ether C-O-C group, but sometimes they are imposing [9 - 12].
Methylene groups appear in the field of 2880 – 2980 cm–1 in the form of weak and medium bands, the absorption band of CF2-H bond lies in the field of higher wave numbers 2995 – 3050 cm–1 because of the presence of electronegative fluorine atoms, very weak intensity is typical for it.
The absorption peak of isolated double bond is within the interval of 1680-1620 cm–1 in allyl polyfluorinated ethers. For synthesized allyl polyfluorinated ethers the absorption bands are observed at 1677 - 1686 cm–1, which is connected to electron withdrawing nature of perfluorinated carbon chain. In case of trichloroallyl polyfluorinated ether the stretching vibrations of double bond are observed at 1621 cm–1(the influence of chlorine atoms). Besides that, at 700-800 cm–1 a strong band appears,it is caused by the stretching vibrations of C-Cl bond.
In case of polyfluorinated ethers of cinnamic alcohol the absorption peak of double bond conjugated with phenyl is observed at 1625 cm–1. Within the range of 1625 - 1575cm–1 aromatic ring vibrations are observed. In the field of 770 - 730 cm–1 and 710 - 690 cm–1 strong bands appear, which are caused by C-H deformation vibrations in monosubstituted aromatic ring. In the field of 3000 cm–1 stretching vibrations of C-H bonds of medium intensity appear. Usually, they are a group of bands. When aliphatic chain is present in molecule besides the aromatic ring the absorption band of C-H aromatic bonds appear as shoulders on the main aliphatic band νC-H.
In 1H NMR-spectra of polyfluorinated ethers the signals of HCF2CF2-group are present in the form of triplets within the range of 5.8 - 6.2 ppm. The triplet splitting is caused by spin-spin couplings of proton with fluorine of HCF2 (JHCF2 =51.6-51.9 Hz) group and weaker couplings with fluorine atoms of neighbouring difluoromethylene group (JHCF2CF2=5.1-5.4 Hz), which corresponds to literature data for HCF2CF2-group [12]. α-Methylene group in H(CF2CF2)nCH2 is appearing in the form of triplet with constant value JHF = 13.2-14.1 Hz, which is explained by spin-spin couplings of protons with fluorine atoms..
In 1H NMR-spectra of synthesized allyl ethers the allyl group appears in the form of typical doublet of O-CH2- with δ=4.38 - 4.47 ppm and multiplet CH2-protons with δ= 5.265 - 5.259 ppm and CH-proton about ppm.
1H NMR spectrum of cinnamic ethers of polyfluorinated ethers is characterized besides all that by the presence of multiplet in the range of 7.0 – 7.3 ppm, belonging to 5 protons of benzene ring.
1H NMR spectrum of trichloroallyl ether of polyfluorinated alcohols is rather simple. Here besides the signals characterizing the polyfluoroalkyl fragment only one single of =CCl-CH2- group is observed.
Experimental
IR- spectra of liquid compounds were taken at "Spekord–M82" instrument in thin film. 1H NMR–spectra were recorded at Mercury-300 (Varian), operating frequency 300 MHz, internal standard tetramethylsilane, solvent – carbon tetrachloride. UV-spectra were recorded in the solution of chloroform at spectrometer SF-2000 ("OKB Spectr" company).
1.Synthesis of 3-(2,2,3,3-tetrafluoro)propoxy-1-propene (CH2=CH-CH2-O-CH2(CF2CF2) H).
A solution of 1.49 g (0.026 mole) allyl alcohol in 30ml chloroform mixed with 3.54 g (0.26 moles) of K2CO3 was placed into three-neck reactor equipped with thermometer, reflux condenser and a stirrer. The reactor was cooled to –15 °C and solution of 5.5 g (0.026 moles) 1,1,3-trihydroperfluoropropylchlorosulphite in 10 ml chloroform was dosed at this temperature. Then the reaction mass was heated up to 30°C and kept stirred for 6 h. After separation from residue and distillation of solvent and unreacted alcohol the product was distilled in vacuum. We obtained 2.92 g (66.2 %) 3-(2,2,3,3-tetrafluoro)propoxy-1-propene, b.p. 95-98 °C/ 15 mm Hg, nD201.3660, d2041.4286. IR-spectrum, ν, cm–1: 529m, 674m, 760s, 794w, 880s, 931s, 983s, 1043m, 1077w, 1108w, 1158 (νC-O-C), 1206 (νCF2), 1326s, 1389m, 1429s, 1489s, 1583w, 1677 (C=C), 2920w, 2989, 3031m (CH2, CH), 3126 (=C-H).
2. Synthesis of 3-(2,2,3,3,4,4,5,5-octafluoro)pentoxy-1-propene (CH2=CH-CH2-O-CH2(CF2CF2)2H).
Obtaining and isolation had been carried out analogously to p. 1. The yield of 40.4%, b.p. 98-100 °C/ 15 mm Hg, nD201.3635, d204 1.4418. Found, %: F 54.29. C8H7F8O. Calculated, %: F 55.88. IR-spectrum, ν, cm–1: 527m, 662m, 759s, 801w, 886s, 945s, 996s, 1038m, 1080w, 1114w, 1165 (νC-O-C), 1207 (νCF2), 1317s, 1385m, 1435s, 1486s, 1554w, 1682 (C=C), 2927w, 2994, 3042m (CH2, CH), 3137 (=C-H).
1H NMR-spectrum, δ, ppm (Spin–Spin Coupling Constants, J, Hz): 6.005 tt (51.9, 5.4) (2H, HCF2); 5.831 multiplet (1H, =CH); 5.265 multiplet (2H, CH2=C); 4.471 d (3.3) (2H, =C-CH2-O); 4.339 t (14.1) (1H, O-CH2CF2).
3. Synthesis of 3-(2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoro) heptoxy-1-propene (CH2=CH-CH2-O-CH2(CF2CF2)3H).
Obtaining and Isolation had been carried out analogously to p. 1. The yield of 86.9 %, b.p. 90 °C/ 6 mm Hg, nD201.3560, d204 1.4816. Found, %: F 60.77. C10H7F12O. Calculated, %: F 61.29. IR-spectrum, ν, cm–1: 523m, 700 s, 751m, 880s, 931s, 1000 s, 1069s, 1094w, 1129w, 1189 (νC-O-C), 1249 (νCF2), 1334m, 1446s, 1497s, 1574w, 1686 (C=C), 2886w, 2997, 3057m (CH2, CH), 3143(=C-H). 1H NMR-spectrum, δ, ppm (Spin–Spin Coupling Constants, J, Hz): 6.017 tt (51.6, 5.1) (2H, HCF2); 5.814 multiplet (1H, =CH); 5.259 multiplet (2H, CH2=C); 4.382 d (3.3) (2H, =C-CH2-O); 3.931 t (13.2) (1H, O-CH2CF2).
4. Synthesis of 3-(2,2,3,3,4,4,5,5-octafluoro)pentoxy-1-phenyl-1-propene (C6H5-CH=CH-CH2-OCH2(CF2CF2)2H).
Obtaining and Isolation had been carried out analogously to p. 1. The yield of 43%, b. p. 55-57 °C/ 1 mm Hg, nD201.4375, d204 1.4587. IR-spectrum, ν, cm–1: 537s, 623m, 691s, 739s, 803s, 889s, 962m, 983w, 1120 (νC-O-C), 1163 (νCF2), 1283s, 1362m, 1394m, 1446s, 1489s; 1621, 1677, 1729 (C=C, Ph), 1823m, 1883m, 1951m, 2869m, 2929 3023, 3057, 3091 (=C-H). 1H NMR-spectrum, δ, ppm (Spin–Spin Coupling Constants, J, Hz): 7.227 multiplet (5H, C6H5); 6.483 d (15.6) (1H, Ph-CH=); 6.126 q (1H, =CH-C-O); 5.930 tt (51.9, 5.4) (1H, HCF2); 4.137 d (6.6) (2H, =C-CH2-O) multiplet (1H, =CH); 5.259 multiplet (2H, CH2=C); 4.382 d (3.3) (2H, =C-CH2-O); 3.931 t (13.2) (2H, O-CH2CF2).
5. Synthesis of 3-(2,2,3,3,4,4,5,5,6,6,7,7-dodecafluoro)heptoxyi-phenyl-1-propene (C6H5-CH=CH-CH2-OCH2(CF2CF2)3H).
Obtaining and isolation had been carried out analogously to p.1. The yield of 32.5%, b.p. 70-73 °C/ 1 mm Hg, nD201.3950, d2041.4821. IR-spectrum, ν, cm–1: 527s, 611w, 679s, 734s, 818s, 903m, 947s, 1000s, 1035m, 1068w, 1169 (νC-O-C), 1237 (νCF2), 1393s, 1435s, 1486s, 1528s, 1570w, 1634, 1718, 1755 (C=C, Ph), 1845m, 1917m, 1988m, 2910m, 2961, 3054, 3087, 3121(=C-H).
6. Synthesis of 3-(2,2,3,3,4,4,5,5-octafluoro)pentoxy-1,1,2-trichloro-1-propene. (CCl2=CCl-CH2-O-CH2(CF2CF2)2H).
4.1 g (0.025 mole) 1,1,2-trichloro-1-propen-3-one (trichloroallyl alcohol) was mixed with 0.1 ml (0.001 mole) of N,N-dimethylformamide in 230 ml of chloroform, cooled down to -10°C and the solution of 7.98 g (0.025 mole) 1,1,5-trihydroperfluoropentylchlorosulphite in 10 ml chloroform was added to mixture at this temperature with stirring. Then the temperature was increased to 30 ºС and stood for 6 h, blowing out with dry air the isolating hydrogen fluoride and sulphur dioxide. The solvent distilled off. Distillation of the residue gave 5.05 g (53%) of ether had been obtained, b.p. 102 - 105 °C/ 1 mm Hg, nD20 1.4180, d204 1.6548. IR-spectrum, ν, cm–1: 506 s, 621w, 674s, 771s (C-Cl), 912s, 974s, 1035m, 1088w, 1150s (νC-O-C), 1212s (νCF2), 1300s, 1379s, 1424s, 1468s, 1621 (C=C), 2906w, 2976 (νCH2), 3008w (CHF2). 1H NMR-spectrum, δ, ppm (Spin–Spin Coupling Constants, J, Hz): 6.002 tt (51.9, 5.1) (1H, HCF2); 4.822 s (2H, =CCl-CH2-O); 4.299 t (13.2) (2H, O-CH2CF2).
References
1. Rakhimov A.I., Nalesnaya A.V., Vostrikova O.V. Zh. Obshch. Khim.,2004, vol. 74,
N 4, p. 967.
2. Rakhimov A.I., Fisechko R.V. Zh. Obshch. Khim.,2007, vol.
77, N 10, p. 1750-1751.
3. Rakhimov A.I., Fisechko R.V. Zh. Obshch. Khim., 2008, vol. 78, N 2, p. 338.
4. Rakhimov A.I, Nalesnaya A.V., Fisechko R.V. Zh. Obshch. Khim,. 2008, 78, N 11. p. 1842-1848.
5. Rakhimov A.I, Nalesnaya A.V ., Fisechko R.V.Pat. 2346926
Russia, С 07 С 43/192, 43/12, 43/225, 43/174. 2009.
6. Rakhimov A.I. Zh. Obshch .Khim., 2010, vol. 80, N 8, p. 1622-1641.
7. Rakhimov A.I, Miroshnichenko A.V., Do Duong Phuong Thao.
Chemistry and Technology of elementorganic monomers and polymeric materials. Volgograd 2010, N 2,
p. 47-49.
8. Do Duong Phuong Thao,Rakhimov A.I, Miroshnichenko A.V.Actual problems of
organic chemistry: Sat. Mater. vserosciyskoy Conf. with the elements of scientific. School for Youth,
6-8 October. 2010 / GOU VPO "Kazan State. Tekhnol. Univ. "- Kazan: Kazan State Technical University,
2010. - p. 20.
9.Bellamy, L. Infrared spectra of complex molecules. – M.: l. L., 1963.
– 590 p.
10. Otto, M. Modern methods of analytical chemistry. - M.: Technosphere, 2006.
– 416 p.
11. Kazitsyna L.A., Kupletskaya N.B. Application of UV, IR, NMR and mass spectroscopy
in organic chemistry textbooks: manual for schools. - M., High. School, 1971. – 264p.
12. Ionin B.I., Ershov B.A. NMR spectroscopy in organic chemistry. - Leningrad: Khimiya, 1967. – 326p.
Recommended for publication by Prof. Alexander I. Rakhimov
Fluorine Notes, 2011, 78, 3-4
