Received: August, 2010
Fluorine Notes, 2011, 77, 7-8
Quantitative determination of sulfonylfluoride group conversion in the copolymer of tetrafluoroethylene with perfluoro(3,6–dioxa -4-methyl-7-octene)sulfonyl fluoride in the process of hydrolysis
S.P.Abramov, A.A.Trofimova, V.G. Barabanov
FCUE Russian Scientific Center "Applied Chemistry", 197198, Russia, St. Petersburg, Dobrolubov av.
14
e-mail: vbarabanov@rscac.spb.ru
Abstract: Applicability of FTIR spectroscopy to quantify the conversion of sulfonylfluoride groups into active groups for ion exchange in the copolymer of tetrafluoroethylene with perfluoro(3,6–dioxa-4-methyl-7-octene)sulfonylfluoride (copolymer F-4SF) in the process of hydrolysis has been investigated. Suggested method takes into account conditions and characteristics of a change of an absorbtion spectrum of F-4SF in the process of hydrolysis, a relative intensity of absorbsion bands and an applicability to the samples of various thickness.
Keywords: FTIR spectroscopy, copolymer, sulfonylfluoride groups, hydrolysise.
Copolymer of tetrafluoroethylene CF2=CF2 (TFE) with perfluoro(3,6-dioxa-4-methyl-7-octene)sulfonyl fluoride CF2=CFOCF2CF(CF3)OCF2CF2SO2F (FC-141) (copolymer F -4SF):
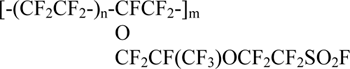
is a precursor of a sulphonic acid form of the copolymer
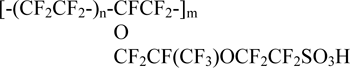
known under the trademark Nafion (E.I. du Pont de Nemours and Co., Inc.) and been of interest as a compound for the manufacture of proton-conducting membranes for various purposes [1]. Nafion is prepared by hydrolysis of F-4SF and conversion of sulfonylfluoride groups into sulphonic acid groups. When processing by alkali salt form Nafion is obtained, for example, sodium (Na-Nafion [2]):
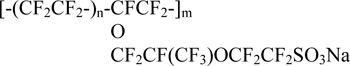
The article is devoted to the investigation of the FTIR spectroscopy applicability for the quantitative determination of sulfonylfluoride group conversion into active for ion exchange groups in the copolymer F-4SF in the process of hydrolysis.
Experimental
The absorption spectra were measured on a FTIR spectrophotometer IR Prestige-21 (according to the technical documentation of "Shimadzu Corporation", Japan). Spectra of solid samples of F-4SF in the form of films were measured relative to the background (air) using the following parameters: apodization - Happ-Genzel, the number of scans - 20, resolution - 4.0 cm-1. Spectra are shown in the wavenumber range from 2200 to 2750 cm-1. To measure the spectra of liquid thin-layer samples FC -141 and FC-151 (CF3CHFOCF2CF(CF3)OCF2CF2SO2F) and a background used polyethylene films as the windows. Spectra are shown in the wavenumber range from 2660 to 2760 cm-1.
Evaluation of the feasibility of absorption bands in the IR spectra of F-4SF and Nafion for the quantitative determination of sulfonylfluoride group conversion and the formation of active groups for ion exchange in the hydrolysis products of F-4SF
IR-spectra of copolymers F-4SF and Nafion are characterized by a number of bands that may be related to the content or the conversion of sulfonylfluoride groups in the copolymer F-4SF. The hydrolysis of copolymer F-4SF must be accompanied by a decrease in the intensity of the bands characterizing sulfonylfluoride group -SO2F and the appearance of bands of -SO3-- group or undissociated -SO3H group.
It is known the method for the control of changes in the concentration of sulfonylfluoride groups -SO2F in the different processes on a peak decrease at ~ 1470cm-1 assigned to the asymmetric-SO2-stretching vibrations in the SO2F group and paired peaks decrease at ~810 cm-1 corresponding to the S-F stretching vibrations of SO2F group [3].
The appearance of bands of stretching modes S=O (at 1410 cm-1) and S-OH (at 910 cm-1) of the undissociated -SO3H group or bands of the –SO3- species : band of symmetric stretching vibrations at 1060 cm-1 and a double band of asymmetric stretching vibrations at 1310-1320 cm-1 [2] as a result of hydrolysis of a sample of the copolymer F-4SF (especially partial) has not been unique for the quantitative control of the process of hydrolysis. In paper [2] it is showed that the appearance or disappearance of these bands depends on the water content in the product of hydrolysis: for example, -SO3H group exists only in the "dry" sample of Nafion, and in samples of Nafion containing water only the -SO3 group is observed. Moreover, the appearance of bands of the undissociated -SO3H group is characteristic of the crystal structure (or parts of the crystal structure of a sample), and in the amorphous structure only the -SO3 group is present [4]. The spectrum of a sodium form Nafion (Na-Nafion) contains only the band of -SO3 group [2]. The need for considering all forms of hydrolysis product of the F-4SF in the spectrum complicates the quantitative determination of sulfonlfluoride group conversion.
The origin of the band at ~ 980 cm-1 in the copolymer F-4SF is connected with ester C-O-C groups [1], which are contained in the monomer of FC-141. Based on the ratio of ether and sulfonylfluoride groups in FC-141, this band may be indirect evidence of the initial concentration of sulfonylfluoride groups in the copolymer F-4SF. But in the case of hydrolysis of sulfonylfluoride groups, ester groups located near the ion-pair with -SO3- and H3O+ groups or an ion of alkali metal have a greater influence from the ion pair than the C-O-C groups located closer to the backbone of the copolymer. It leads to the appearance of a shoulder (the second peak) next to the dominant peak at ~ 980 cm-1 [1]. The band corresponding to the ester groups may feel the influence due to the formation of hydrogen bonds between oxygen atoms and the protonated particles [2] (H3O+ or larger associates). Thus, the use of this band for normalization when determining the conversion of sulfonylfluoride groups is associated with the need to stabilize the environment of ester groups.
For normalization of the sample quantity for measurement it can be used the bands characteristic for the carbon backbone of the copolymer F-4SF containing CF2 group. In paper [2] it is mentioned that the bands of Nafion at 1220 and 1155 cm-1 related to the stretching vibrations of CF groups do not have a significant influence from the adsorbed water. The bands at 1145 cm-1 and 1200 cm-1 similarly interpreted in paper [5] related to the stretching vibrations of CF2 group (symmetric and asymmetric respectively) according to the authors are not affected by adsorbed water and may experience only a small change in the spectral profile during hydration, which may be associated with the reorganization of the crystalline domains. Thus, it is possible to use of the considered bands for normalization in the measurements of hydrolyzed samples of F-4SF. In the spectrum there is also a doublet at 640-625 cm-1 that arises from the CF2 groups wagging in a normal molecular helical conformation and from one in a reversed molecular helix (helix reversal) along the F-4SF and Nafion backbone which is very sensitive to a temperature change, and next to the SO2F group (at 606 cm-1) in F-4SF [3,6]. The temperature dependence of the doublet and the proximity of the SO2F group make it less suitable for normalization in the quantitative determination of the SO2F groups in the process of hydrolysis of F-4SF.
Investigated bands in the IR absorption spectra of copolymer F-4SF and Nafion can be used to determine the conversion of sulfonylfluoride groups in the sample of F-4SF in the process of hydrolysis taking into consideration of the investigated features of these bands in the spectrum and their intensity. Most bands are too intense and only suitable for measurements in the films of the samples F-4SF with a thickness much smaller than 0.1 mm. Based on the technological features of the film preparation from the copolymer F-4SF and its further transformation it is of interest to develop a method for the quantitative determination of sulfonylfluoride groups in the process of hydrolysis of the films of F-4SF with a thickness of 0.1mm. Previously [7] it has been described the method for the quantitative determination of sulfonylfluoride groups in the copolymer F-4SF using relatively weak analytical bands at 2703 cm-1 and 2450 cm-1 (the shoulder in a group of bands) in the IR absorption spectrum of the copolymer F-4SF (Fig. 1), which is applicable for the films with large thickness, in particular 0.1 - 1 mm, typical for the films obtained by extrusion method.

Fig.1. IR absorption spectra of copolymer F-4SF with a different percent of conversion of sulfinylfluoride groups in the range of analytical bands at 2703 cm-1 and 2450 cm-1: initial copolymer - the spectrum A (conversion 0%), partially hydrolyzed copolymer - specrum B (conversion 61%). D - optical density (rel. units.), ν - the wavenumber (cm-1).
Band at 2703 cm-1 in the absorption spectrum of F-4SF (Fig. 1) being absent in the spectrum of PTFE and demonstrating a good correlation dependence on the sulfur content in the samples of F-4SF, used as a measure of the content of sulfonylfluoride groups of the monomer of FC-141 in the copolymer F-4SF [7]. When considering the spectrum of FC-141 it is revealed a weak band with maximum absorption at 2700-2702 cm-1 (Fig. 2).
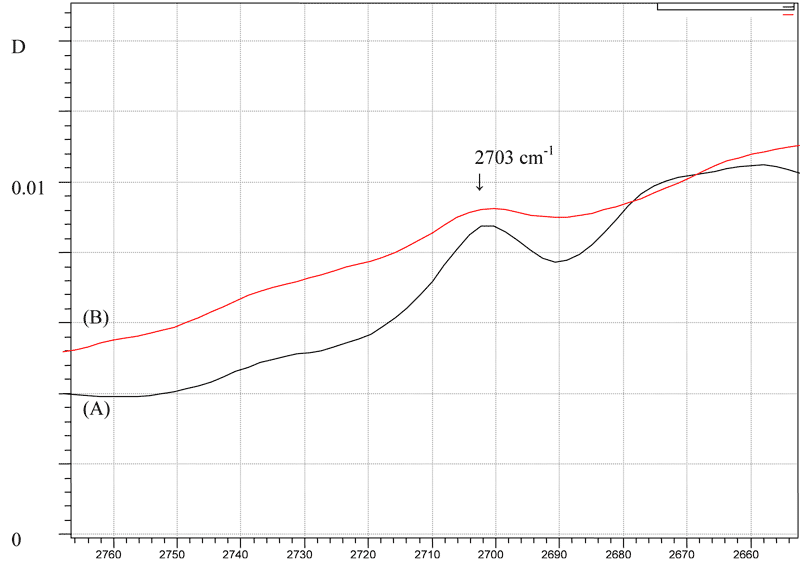
Fig.2. IR absorption spectra of FC-141 (spectrum A) and FC-151 (spectrum B) in the range of the analytical band at 2703 cm-1. D - optical density (rel. units.), ν - the wavenumber (cm-1).
In the same range of the spectrum it is revealed a weak band of FC-151 also having a sulfonylfluoride group in its structure (Fig. 2). With regard to the differences in intramolecular and intermolecular interactions in the FC-141 and F-4SF (A molecule of FC-141 is solvated by molecules of FC-141 and F-4SF has a crystalline or amorphous structure) the examined band at 2700-2702 cm-1 apparently can be identified with the band at 2703 cm-1 of the copolymer F-4SF. Based on the properties of the band at 2703 cm-1 : a good correlation between the intensity of the band and the sulfur content in the samples of F-4SF [7] and a decrease in the intensity of the band in the process of hydrolysis (Fig. 2), its origin can be associated with the involvement of the sulfonylfluoride group in the formation of the IR spectrum of F-4SF. It allows the use of the band at 2703 cm-1 to estimate the percent of conversion of sulfonylfluoride groups in the hydrolysis of copolymer F-4SF for the relatively thick films of the samples with the dimension of thickness exceeding 0.1 mm and a small conversion of sulfonylfluoride groups.
The bands of the absorption spectrum in the wavelength range of 2745 - 2200 cm-1 (Fig. 1) are characteristic for the F-4SF and, excluding the band at 2703 cm-1, for polytetrafluoroethylene (PTFE) [7]. The absorption bands in this range of the IR-spectrum of PTFE are interpreted in paper [8] as the combination bands, relating to the CF2 groups in the chains of PTFE. А set of bands in this spectral range in the samples of Nafion is interpreted as in paper [9]. Taking into account that the copolymer F-4SF has a backbone consisting of CF2 groups, the analytical signal with the wavenumber 2450 cm-1 (the shoulder) in this specific range of the spectrum has been used to normalize the amount of the copolymer F-4SF ( a sample thickness) at measurements [7]. With regard to the conditions of the appearance in the spectrum, the analytic signal can be used for the normalization of the sample thickness of F-4SF in the process of hydrolysis.
Taking into account the examined features of IR absorption spectra of copolymers F-4SF and Nafion the control for the sulfonylfluoride group conversion during hydrolysis of F-4SF can be carried out using analytical signals at 2703 cm-1 and 2450 cm-1 for the samples with the dimension of thickness exceeding 0.1 mm and a small conversion of sulfonylfluoride groups. At the final stage of hydrolysis of F-4SF in order to assess the completeness of the conversion of sulfonylfluoride groups, it is advisable to use a more intense band of -SO2F group (at ~1470cm-1 in paper[3] and at 1467cm-1 in this paper) instead of the band at 2703 cm-1.
In the case of the acid form of Nafion a well-defined infrared absorption spectrum becomes less structured when increasing of the equilibrium water amount [2] through the formation of a set of proton-consisting associates. In its turn it makes difficult to carry out measurements in the range of the analytical bands at 2703 cm-1 and 2450 cm-1. In the case of the sodium form Na-Nafion bands of weakly adsorbed water (coordinated with Na+ and been similar of liquid water) are only present in the spectrum [2]. Thus, the assessment of the conversion of sulfonylfluoride groups in the F-4SF is advisable to conduct at the stage of the formation of a salt form of the product of hydrolysis.
Methodological principles of the control for the conversion of sulfonylfluoride groups and the formation of the active ion exchange groups in the hydrolysis of F-4SF
The concentration of sulfonylfluoride groups in the unhydrolized copolymer F-4SF can be defined as the molar mass of the copolymer per one mole of the sulfonylfluoride group [equivalent mass (EM)] [7]:
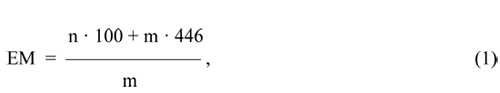
where n - number of moles of TFE, forming the structure of a sample of F-4SF (mol);
m
– the number of moles of FC-141, forming the structure of the sample of F-4SF (mol), 100 - the
molar mass of the TFE (g *mol-1), 446 - the molar mass of FC-141 (g*mol-1).
The method of determining of the EM of the unhydrolized copolymer F-4SF was described in paper [7]. EM was determined after measuring of the optical density values for the corresponding analytical bands in the infrared absorption spectrum of a sample of F-4SF:

where D2450 and D2703 - the values of absorption (the value of analytical signals) corresponding to the analytical bands at 2450 and 2703 cm-1 relative to a baseline (optical density, rel. units.); K - proportionality factor (283.2 g*mol-1), calculated from the correlation between the ratio of analytical signals in the infrared absorption spectrum and the sulfur content in the samples of F-4SF [7].
If we denote the equivalent mass of the F-4SF with partially hydrolyzed sulfonylfluoride groups EMh, and the equivalent mass of the initial F-4SF - EM, the concentration of unhydrolyzed sulfonylfluoride groups (C,%) will be determined by the equation:
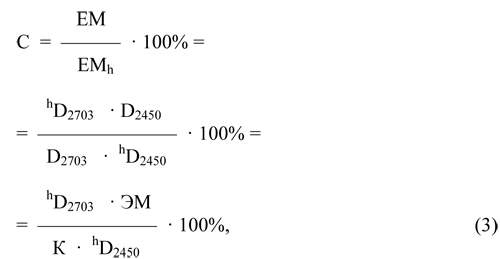
where hD2703 and hD2450 - the values of the absorption bands corresponding to 2703 and 2450 cm-1 relative to a baseline (optical density, rel. units.) for a partially hydrolyzed sample of F-4SF.
In the case of a low concentration of unhydrolyzed sulfonylfluoride groups (high values of the EMh at a high conversion during hydrolysis) or in the case of thin film samples of F-4SF small values of hD2703 will lead to the large miscalculations in the concentration determination. In this case it is advisable to pass to the expression for the concentration of sulfonylfluoride groups through the optical density for more intense absorption band of -SO2F group at 1467 cm-1 (hD1467). For samples of F-4SF which thickness and equivalent mass allowed to carry out the simultaneous correct measurement of the intensity of the absorption bands at 1467 cm-1 and 2703 cm-1 it has been determined by the ratio of the intensities of these bands: D1467/D2703=126 (mean-square deviation is +/-6) where D1467 and D2703 - the values of the absorption corresponding to the bands at 1467 and 2703 cm-1 relative to a baseline (optical density, rel. units).
In this case the expression (3) for the concentration of sulfonylfluoride groups can be transformed as:
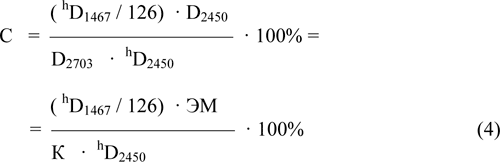
The total concentration of active for the ion exchange groups: -SO3H, -SO3 and -SO3Na (a special case of a salt form) in the hydrolysis products of F-4SF can be expressed as a molar mass of polymer per one mole of the -SO3 group (in the form of equivalent mass EMSO3(g*mol-1)), regardless of its form of presence in the copolymer (as-SO3H,-SO3- or, for example,-SO3Na). EMSO3 in the hydrolysis products of F-4SF taking into account the equivalent mass of the initial F-4SF (EM) and the concentration of unhydrolyzed sulfonylfluoride groups (C) in the product of hydrolysis is given by:
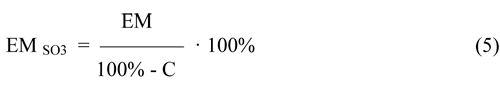
The proposed method allows to determine the concentration of active centers for the ion exchange in the hydrolysis products of F-4SF regardless of the presence of these active sites (as a group -SO3H, -SO3- or, for example,-SO3Na) in the hydrolysis products of F-4SF.
Conclusions:
1.The determination of sulfonylfluoride groups in the copolymer F-4SF by FTIR spectroscopy in the process of hydrolysis is advisable to conduct at the stage of the formation of a salt form (for example, a sodium form) of the hydrolysis product.
2. Bands at 2703 cm-1 and at 2450 cm-1 (the shoulder in a group of bands) in the IR absorption spectrum of the copolymer F-4SF can be used to quantify sulfonylfluoride groups in the copolymer F-4SF in the process of hydrolysis for the samples with the dimension of thickness greater than 0.1 mm and a small conversion of sulfonylfluoride groups.
3. To determine the amount of sulfonylfluoride groups in the F-4SF with a small thickness of the sample (less than 0.1 mm) and (or) with a high conversion of sulfonylfluoride groups it is proposed to use a more intense band at 1467 cm-1 instead of a band at 2703 cm-1 taking into account the ratio of band intensities D1467/D2703 equal 126.
4. The initial equivalent mass (EM) of the copolymer F-4SF and the concentration of unhydrolized sulfonylfluoride groups (C) in the hydrolysis products of F-4SF determine the total concentration of active for the ion exchange groups, regardless of the form in which they are present (-SO3H, -SO3- or in the salt form (e.g.-SO3Na)).
References
- Mauritz K.A., Moore R.B. // Chem. Rev. 2004. V. 104. N 10. P. 4535-4585.
- Buzzoni R., Bordiga S., Ricchiardi G., Spoto G., Zecchina A. // J. Phys. Chem. 1995. V.99. N 31. P. 11937-11951.
- Greso A.J., Moore R.B., Cable K.M., Jarrett W.L., Mauritz K.A. // Polymer. 1997. V. 38. N 6. P. 1345-1356.
- Ludvigsson M. Materials for future power sources: Acta Univ. Ups. Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 560. Uppsala ISBN 91-554-4789-9. 2000. 57 p.
- Grosmaire L., Castagnoni S., Huguet P., Sistat P., Boucher M., Bouchard P., Bebin P, Deabate S. // Phys. Chem. Chem. Phys. 2008. N 10. 1577-1583.
- Hsu W.Y. // Macromolecules. 1983. V. 16. N 5. P. 745-749.
- Abramov S.P., Trofimova A.A., Barabanov V.G. // Zhurn.prikl.khimii. 2009. V.82.N 12. 2043-2047.
- Moynihan R.E. // J. Am. Chem. Soc. 1959. V. 81. P. 1045-1050.
- Basnayake R., Wever W., Korzeniewski C. // Electrochimica Acta. 2007. V. 53. P. 1259-1264.
Recommended for publication by V. Kornilov
Fluorine Notes, 2011, 77, 7-8
