Received: July, 2011
Fluorine Notes, 2011, 77, 5-6
THE FLUOROALIFATIC SULFOCHLORIDE DERIVATIVES OBTAINING BY A METHOD OF THE PHOTO-CHEMICAL SULFOCHLORINATION
O.S.Andrienko1,2, V.V.Zhuk1, T.D.Malinovskaya1, N.F.Mingalimov1, V.I.Sachkov1,3, V.A.Yanovsky1
1) Isolated Structural Division "Siberian Physico-technical Institute Named of the academician
V.D.Kuznetsov" of Tomsk State University, 634050, Russia, Tomsk, Novosobornaya sq.1, tel. (3822)413799,
e-mail: its@spti.tsu.ru
2) V.E. Zuev Institute
of Atmospheric Optics Russian Academy of Sciences, Siberian Branch, 6340211, Russia, Tomsk, Academician
Zuev sq. 1
3) Institute for Problems of Chemical and Energetic Technologies Russian
Academy of Sciences, Siberian Branch, 659322, Russia, Altai krai, Biysk, ul. Socialisticheskaya,
1
Abstract: In this study we describe the method of the fluoroalifatic sulfochloride derivatives obtaining by a method of the photo-chemical sulfochlorination. Optimal conditions for synthesis were found and the reaction of difluoromethane, Cl2 and SO2 was studied.
Keywords: Fluoroalifatic sulphoacids, sulfochlorination, fluoroalkane sulphochlorides.
The fluorinated aliphatic sulphoacids and their derivatives are widely used in the synthesis of medicinal substances, pesticides, as acid catalysts of various organic reactions, as biologically active substances [1,2] and, also in agriculture [3]. However, existing now, the methods of synthesis of these compounds possess a number of essential lacks, such as, low yields, synthesis severe conditions, complexity of selection that causes high cost of the products in the world market and considerably are limited by their production. For example, the most developed method of obtaining of difluoromethanesulphoacids is sulphonation of Freon-22 by sodium sulphite. Reaction is carried out in an autoclave within 20h at temperatures 120-150°C and high pressure from 80 to 150 atm., provided that the main product yields is no more than 50 % [2].
Therefore the research of essentially new synthesis route of the fluorinated aliphatic sulphoacids is an actual scientific and technical task.
We were first carried out sulfochlorination of fluoroalifatic hydrocarbons.
The purpose of this work consisted in creation of a new synthesis route for fluoroalkane sulphochlorides obtaining from fluoroalkanes, chlorine and sulfurous anhydride.
The reaction was carried out in the flowing quartz photochemical reactor equipped with a jacket. The sulfochlorination reaction was carried out both in gas and liquid phase, carbon tetrachloride used as a solvent. The temperature ranged from -20 to +50°C. Under the influence of UV radiation, the radical reaction of difluoromethane, Cl2 and SO2 take place. UV lamps (λ = 254 nm, λ = 310 nm) are used as a source of UV radiation.
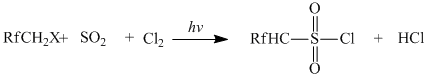
where Rf = F, CF3, C2F5; X = H, F
The experimental results of obtaining of fluoroalkane sulphochlorides are presented in Table 1.
Table 1. Conditions and yields of the reaction of direct photo-chemical sulfochlorination of alifatic hydrocarbons
|
# |
Initial fluoroalkane |
The composition of the reaction mixture, mole to 1 mole fluoroalkane |
T, °C |
Yield, % |
||
|
Rf |
X |
Cl2 |
SO2 |
|||
|
1 |
F |
F |
0.5 |
1.5 |
25 |
46 |
|
2 |
F |
F |
1 |
1.5 |
25 |
44 |
|
3 |
F |
F |
1 |
2 |
25 |
40 |
|
4 |
F |
F |
1 |
3 |
25 |
42 |
|
5 |
F |
F |
1 |
1.5 |
0 |
36 |
|
6 |
F |
F |
1 |
1.5 |
40 |
31 |
|
7 |
F |
H |
1 |
1.5 |
25 |
60 |
|
8 |
CF3 |
F |
1 |
1.5 |
25 |
43 |
|
9 |
CF3 |
H |
1 |
1.5 |
25 |
51 |
|
10 |
CF3 |
H |
0.75 |
2 |
25 |
55 |
|
11 |
CF3CF2 |
H |
0.75 |
1.5 |
25 |
45 |
|
12 |
CF3CF2 |
H |
1 |
1.5 |
25 |
43 |
|
13 |
CF3CF2CF2 |
H |
1 |
1.5 |
25 |
30 |
|
14 |
CF3(CF2)6 |
H |
1 |
1.5 |
25 |
24 |
As can be seen from the results shown in Table 1 the most effective carrying out the reaction is at room temperature and the ratio of reagents: fluorohydrocarbons: Cl2: SO2 = 1:1:1.5.
A new method of fluoroalkansulphochlorides synthesis is offered. The structures of all obtained compounds were proved by NMR spectroscopy 1H and 13C, IR spectroscopy and mass spectrometry.
The research was supported financially by FTP "Scientific and scientific and pedagogical shots of innovative Russia" for 2009-2013 (Projects #02.740.11.0257 and # P74).
References
- Filler R., Kobayashi Y., Yagupolskii Y. L. Organofluorine Compounds in Medicinal Chemistry and Biological Applications. – Amsterdam: Elsevier, 1993.
- Ojima I., McCarthy J. R., Welch J. T. Biomedical Frontiers of Fluorine Chemistry. – Washington, DC: Eds, 1996.
- Banks R. E. Fluorine in Agriculture. – Sale, Cheshire, U.K: Fluorine Technology Limited, 1995.
Recommended for publication by S. Igoumnov
Fluorine Notes, 2011, 77, 5-6
