Received: March, 2011
Fluorine Notes, 2011, 77, 3-4
EFFECT OF POLYFLUOROALKYL GROUPS IN COPOLYMER OF ACRYLAMIDE-ACRYLATE ON PROPERTIES OF ITS DULITE AQUEOUS SOLUTIONS
Rakhimov A.I, Vershinin D.A, Miroshnichenko A.V., Rakhimova O.S.
Volgograd State Technical University, Russia, 400131, Volgograd, Lenin Prosp., 28
e-mail: organic@vstu.ru
Abstract: It has been found that in dilute aqueous solutions of the copolymer of acrylamide- sodium acrylate its conditional viscosity reduces in 5.9 times to 0.1% solution and in 5.2 times to 0.3% solution and a freezing point of the solution drops to minus 20 ° C. It allows us to recommend an application of dilute aqueous solutions of polyfluorinated copolymers in various fields of technology.
Keywords:Polyfluoroalkylchlorosulphites, copolymer of acrylamide-sodium acrylate, polyfluoroalkyl ester, sodium acetate.
Previously [1-3] it was found that polyfluoroalkylchlorosulphites were unique polyfluoroalkylsulfonating reagents whose action on carboxylic acid salts results in formation of esters of polyfluorinated alcohols.
In this paper (by the example of 1,1,5-trihydroperfluoropentylchlorosulphite) it has been shown that this reaction can be used for the introduction of a polyfluoroalkyl group in the copolymer of acrylamide-sodium acrylate:

Polyfluoroalkylation of sodium acrylate groups in the copolymer has been carried out by 1,1,5- trihydroperfluoropentylchlorosulphite by the methods described in [2,3].
The molecular weight of the initial copolymer is 1.2·106, and the ratio of links in the macromolecular chain is defined as 60% acrylamide and 40% sodium carboxylated ones.
By a method of direct potentiometry with a fluoroselective electrode it was determined the fluorine content in the sample (after combustion and titration) which was 3.7% (mass). The IR spectrum of a polyfluoroalkylated copolymer in contrast to the initial copolymer has an intense absorption band at 1760 cm-1. This indicates that the reaction of 1,1,5- trihydroperfluoropentylchlorosulphite with a sodium carboxylated group of the copolymer proceeds by analogy with the sodium salts of monocarboxylic acids [2] and sodium salts of oligomers of ε-aminocaproic acid [3].
The content of fluorine calculated on an oktafluoropentyl fragment introduced into the copolymer is 0.013% (mass). Consequently, it was estimated that approximately 6.6% (mass) of sodium carboxylated groups entered into the reaction of polyfluoroalkylation which corresponds to z≈300.
Measurement of a conditional viscosity of aqueous solutions of the copolymer was carried out with a three-horn glass capillary viscometer (VPZH-1) at 22°C, the bulk of the sample solution was 25 ml and diameter of a capillary was 1.52 mm. The concentration of aqueous solutions of copolymer was 0.1% (mass) and 0.3% (mass) (Table 1).
Table 1. Effect of concentration of aqueous solutions of copolymer and its polyfluoroalkyl derivative on viscosity.
|
Concentration, % |
Conditional viscosity, sec. |
|
|
Initial oligomer |
Polyfluoroalkylated oligomer |
|
|
0.3 |
83 |
16 |
|
0.1 |
59 |
10 |
|
water |
4 |
As can be seen from Table 1, conditional viscosity of 0.1% (mass) aqueous solution with polyfluorinated fragments of the copolymer decreases of 5.9 times, and in the case of 0.3%(mass) aqueous solution of 5.2 times. It is obvious that the introduction polyfluoroalkyl groups in a macromolecule of the copolymer has a significant impact on the structure of water clusters in comparison with non-fluorinated copolymer [4]. This can be explained by the fact that the introduction in a macromolecule of a copolymer about three hundred H(CF2CF2)2CH2-fragments which have hydrophobic properties, reduces the molecular weight of the cluster structures formed with the participation of functional groups of the copolymer.
The most important property of aqueous solutions is their freezing point. In this context we compared the effects of acetate groups by the example of sodium acetate (recently widely used as an antiglaze substance) and investigated copolymers with sodium chloride and calcium chloride. In Table 2 they are shown the freezing points of aqueous solutions of these salts and copolymers.
Table 2. Effect of the composition of aqueous solutions on the freezing point.
|
Table 2. Effect of the composition of aqueous solutions on the freezing point.
|
||||||||||||||||||||||||||||||||||
As can be seen from Table 2, sodium acetate and sodium formate make the greatest effect on the reduction of the freezing point at low concentrations, and the presence of a large number of sodium carboxylated groups in the macromolecule of the copolymer significantly enhances the effect - the freezing temperature drops to minus 18°C. The introduction of polyfluoroalkyl groups has a little effect on the reduction of the freezing point of water solution (minus 20°C).
To estimate the impact of sodium carboxylated groups we have carried out the analysis of possible cluster structures formed with these groups in comparison with metal chlorides.
For that, the electronic structures of dimers and tetramers of water, sodium acetate, formate and chloride, as well as associates of sodium acetate, formate and chloride with the water dimer, which can be formed in process of the interaction of salts with water clusters have been analyzed by the quantum-chemical method ab-initio in the basis 6-31G.
Nonempirical method allows to take into account all interactions between electrons and nuclei in molecules. Analysis of the electronic and geometric structure of complexes based on sodium acetate and formate with the water dimer has shown that the structures of the complexes are identical.
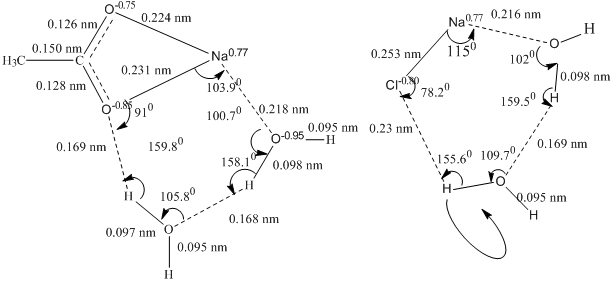
Fig. 1. Complex of sodium acetate and sodium chloride with two molecules of water.
In associates of sodium salts with the water dimer (elementary part of the cluster) six-membered structure between the contact ion pair of a sodium salt molecule and the water dimer is formed (Figure 1). A comparison of atomic charges, bond lengths, bond angles, rotation barriers of the hydrogen atoms around the bonds in the initial dimers and complexes have been carried out as well as determined the total energy of the formation of complexes from the initial structures.
Prolongation of the stretching HO-bonds from 0.096 nm to 0.098 nm has been observed in the complexes in comparison with the free dimer. Bond angle in the dimer is 180°, and in the structure with NaCl and salts of carboxylic acids it decreases to 159.5° and 158.1° respectively.
Changes in the hydrogen and oxygen atomic charges of both water molecules in the structure of the complex are important details about the polarization of the bonds, leading to changes in bond angles of the dimer. In sodium chloride complex positively charged sodium reacts with the oxygen atom of the dimer which leads to a decrease in oxygen negative atomic charge to -0.95 (in the free dimer was -0.88). At the same time valence HO- bond has become polarized. As a result oxygen removed in the hydrogen bond is also becoming more negative (the charge is reduced from -0.83 to -0.91). The hydrogen atoms of water molecules in the complex, due to the polarization of the bonds are gaining more positive charge. Hydrogen associated with chlorine acquires a charge +0.48 (was +0.43) and the hydrogen atom associated with a water molecule in the dimer the charge varies from +0.47 to +0.54. The atomic charges in the complex of sodium acetate with two molecules of water are increasing rather more: the hydrogen atomic charge associated with the oxygen of carboxylate group rose to +0.54 (was +0.43), and the hydrogen atom involved in hydrogen bonding with other water molecule has charge equal +0.54. Clearly, the large polarization of water dimer bonds occurs in complexes with sodium acetate and sodium formate.
Energy gain of complexation was estimated by the formula:
ΔEcomplex = ∑Einitial - ∑Ecomplex.
And although the energy values differ slightly, according to the stability of the complexes they can be arranged in series:
ΔEHCOONa (-38.4) > ΔECH3COONa (-38.3) > ΔENaCl (-38.1) (kcal/mol)
Moreover, the formation of cyclic tetramer of water from the two dimers is energetically less favorable (ΔEtetramer=-26.7 kcal/mol), which indicates the possibility of the decomposition of clusters with the formation of stable salt complexes.
The calculation of rotation barriers of the hydrogen atom associated with chlorine of sodium chloride complex about the hydrogen H...O-band has a negligible amount (2.8 kcal/ mol). The magnitude of the rotation barrier increases in almost two times in HO-group associated with the oxygen of acetate and formate groups. These differences in energy rotation barriers play a significant role at low temperatures, promoting to the embedding of more stable complexes in the cluster structure and making an impact on the polarity of the spatial polyclustered structure of water.
This analysis explains the significant impact a great number of sodium carboxylate groups in the copolymer of acrylamide-sodium acrylate, which dilute solutions have a low freezing point and low conditional viscosity in the case of polyfluoroalkylated derivatives that allows us to recommend them for the application in various fields of technology.
References
- Rakhimov A.I., Vostrikova O.V. Synthesis and properties of polyfluoroalkylchlorosulphites // Fluorine compounds. Chemistry, technology, applications. : collection of scientific papers (Jubilee Collection). Federal State Unitary Enterprise Russ. Nauch. Tsentr "Applied Chemistry ". - St.- Petersburg. - 2009. - pp. 314-321.
- Rakhimov A.I., Nalesnaya A.V., Vostrikova O.V., Rakhimova N.A., Babushkin A.S. Reaction of carboxyl-containing compounds and their salts with polyfluoroalkylchlorosulphites // News of the Volgograd State Technical University. Chemistry and Technology of Elementorganic monomers and polymers. - 2009. - N 2. - pp. 30-32.
- Storozhakova N.A., Rakhimov A.I., Nalesnaya A.V. Synthesis of polyfluoroalkylated esters of ε-aminocaproic acid oligomers // News of the Volgograd State Technical University. Chemistry and Technology of Elementorganic monomers and polymers. - 2007. - N 1. - pp. 39-52.
- Zenin S.V., Tyaglov P.V. Hydrophobic model of the structure of molecular associations in water // Zhurnal fizicheskoi khimii. - 1994. - V. 68. - N 4. - pp. 636-641.
Recommended for publication by Prof. Alexander I. Rakhimov
Fluorine Notes, 2011, 77, 3-4
