Received: August, 2010
Fluorine Notes, 2011, 76, 7-8
ELECTROCHEMICAL AND DIFFUSION CHARACTERISTICS OF MODIFIED PERFLUORINATED MEMBRANES MF-4SK
S.V. Timofeev 1, N.A. Kononenko 2., L.P. Bobrova 1, N.P. Berezina 2, E.K Luticova 3, S.B. Dolgopolov 2
1JSC "Plastpolymer", Saint-Petersburg, Russia,
2 Kuban State University, Krasnodar,
Russia
3National Research Centre "Kurchatov Institute", Moscow, Russia
e-mail: stimof@yandex.ru
Abstract:We present the results of our studies on the modified perfluorinated ion-exchange membranes MF-4SK used for solid polymer electrolyte in water electrolysers. Their modification was carried out by the insertion up to 20 mass% of organic or inorganic hydrophilic substances: polyvinyl alcohol, sulphated polysulfone, acid zirconium phosphate into membranes produced by extrusion or by casting. It was shown that such introduction of modifiers increases the membrane hydrophilicity and conse-quently its electric conductivity in protonic form. It resulted in 1.5 – 2.0 times decrease of those membranes' diffusion permeability for hydrogen as to compare with reference standard membranes. High chemical resistance of the membranes modified with acid zirconium phosphate was demonstrated us-ing an express-method for its estimation.
Keywords:perfluorinated membrane, modification, electric conductivity, gas diffusion
Designing of water electrolysers with solid polymer electrolyte (SPE) for hydrogen generation is among the priorities in the development of alternative power sources. The central fragments of those units are proton-conducting membranes [1, 2]. Despite large success achieved in the synthesis and modification of ion-exchange polymer membranes [3-8], the search for membrane materials with optimal properties remains actual, particularly taking into account the generation of pure hydrogen at high pressure, up to 13MPa. The most important demands made on the membranes for solid-polymer water electrolysers are their chemical stability, including their resistance to the action of oxidizers at high temperature, high electric conductivity and low gas permeability. Currently the only commercially available membranes that offer this set of properties are perfluorinated sulfo-cation membranes Nafion™ developed by "Dupont" (USA) in the middle of XX century [9]. The same type of membranes is being developed in Russia within recent 30 years by JSC "Plastpolymer" [10-11] and FSUE RSC "Applied Chemistry" (St.Petersburg, Russia) under name of MF-4SK. In an effort to improve electro-osmotic and gas diffusion properties of the membrane intensive a search was undertaken for the methods of perfluorinated membrane modification by the insertion of various water-binding additives into their structures [3, 7, 12]. Some polymer hydrogels, hydrocarbon ion-exchangers with high exchange capacity, or some mineral substances (e.g., silicon oxides, aluminium oxides, or acid zirconium phosphates) are applicable for such additives. The main problem is the search for a compromise between stable water-content, gas permeability, conductivity and other electro-transport characteristics of those membranes on one hand, and their chemical resistance on the other hand.
In this article we are reporting the manufacture of MF-4SK perfluorinated membranes, modified with various hydrophilic substances, and our study on their electrochemical and gas-diffusion properties regarding the use of those membranes in water electrolysers.
EXPERIMENTAL
The investigation is concerned withMF-4SK perfluorinated membranes, modified with hydrogels (polyvinyl alcohol), sulfated polysulfone, or acid zirconium phosphate (Table 1). In this study we used either membranes produced by the extrusion of melt original polymer followed by the hydrolysis of resulting films, or cast membranes manufactured using the solution of hydrolyzed membrane polymer in dimethylformamide.
The membranes modified with acid zirconium phosphate (MF-4SK/ZrP) were prepared on the basis of extrusion membranes. Prior to processing MF-4SK membranes in Н+-form were kept for 24 hours in aqueous solution of zirconium chloroxide ZrOCl2*8H2O. This being done the membranes were washed with distilled water and placed into phosphoric acid solution for sedimentation of acid phosphate zirconium Zr(HPO4)2.
Table 1. Objects of research
|
Perfluorinated membrane MF-4SK |
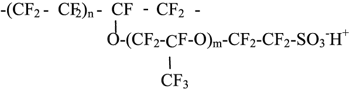 |
| Modifiers | |
| Polyvinyl alcohol (PVA) | 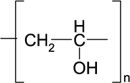 |
|
Sulfated polysulfone (SPS) |
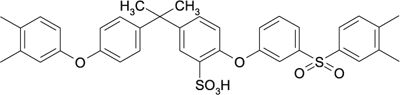 |
| Acid zirconium phosphate (ZrP) | Zr(HPO4)2 |
Casted membranes modified with organic substances were produced by the method of solution casting. To do this we mixed in desired proportion preliminary prepared solutions of the modifier and hydrolyzed membrane polymer in dimethylformamide at room temperature and filtered the resulting solution under vacuum through a capron filter; then we poured it onto a glass with limiting frames in preliminary calculated quantity sufficient for the manufacture of membranes of desired thickness, d, and placed it into a thermostat in order to remove solvent. Those PVA-modified membranes were further treated with furfurol that performs as a crosslinking agent for polyvinyl alcohol, in order to prevent outwashing of PVA from the membranes in contact with water or aqueous electrolyte solutions. The resulting modified membranes were treated with aqueous nitric acid solution, washed with distilled water at room temperature, and then treated with distilled boiling water. The quantity of modifiers added to the membranes is shown in Table 2.
Table 2. Physico-chemical characteristics of the membranes under study.
|
Sample # |
Membrane |
d·106, m |
Q, mmole/g |
W, % |
nm, |
|
1. |
MF-4SKexp, produced by extrusion of melt sample |
150±10 |
0.93 |
25 |
15 |
|
2. |
MF-4SKcast, produced by casting of solution |
140±5 |
1,01 |
32 |
18 |
|
3. |
MF-4SK/ ZrP (20%) |
140±10 |
2.84 |
35 |
7 |
|
4. |
MF-4SK/ ZrP (2,3%) |
140±5 |
1,20 |
32 |
15 |
|
5. |
MF-4SK/SPS (10%) |
140±10 |
1,05 |
36 |
19 |
|
6. |
MF-4SK/PVA (10%) |
140±10 |
0.90 |
43 |
27 |
A set of standard certified techniques was applied to determine the physico-chemical characteristics both of initial membranes and of resulting modified samples [13-14]. The exchange capacity (Q, mmole/g) was determined by titration of Н+-ions released in the process of those membranes alkali neutralization. Humidity percentage (W) was determined by gravimetry as water-to-dry mass ratio. The sample specific electric conductivity (κm, S/m) was calculated on the basis of active part of the membrane impedance measured by the mercury-contact method at the frequency of alternating current about 200 kHz. To determine the integral coefficient of diffusion permeability (Pm, m2/s) the electrolyte solution was made to diffuse through the membrane into clean water under continuous conductometric monitoring of the electrolyte in-chamber concentration.
To estimate the membrane properties we applied also the method of membrane voltammetry. Recently some authors [15-17] proved the applicability of this method in the research of electrochemical properties of perfluorinated membranes modified by various methods: insertion of organic counter-ions, variation of the conditions for chemical conditioning, polymerization of aniline in the membrane matrix, etc. Voltammetric curves were measured in a cell with platonic polarizing and silver-chloride measuring electrodes at the scan velocity of polarizing current 1·10-4 A/s under the conditions of laminar hydrodynamic regime [15]. To characterize the membranes we used: slope of ohmic district (i/ΔE), density of limit electro-diffusion current (ilimit, A/m2), potentials corresponding to the limit (ΔElimit, mV) and super-limit (ΔEcr, mV) state, and the size of plateau of limit current (Δ, mV). The limitation of characteristic points was conducted following the tangent method with the help of "Microsoft Excel" software.
The membrane electric conductivity and diffusion characteristics were determined in НCl solutions, because proton transfer governs the membrane performance in various electrochemical plants, including water electrolysers, or in NaCl solution that makes the mineral basis of natural water, industrial and physiological solutions, and applied traditionally in the comparison of various membrane type characteristics. Those experiments were conducted under isothermal conditions at 25°C, for every sample the error of all its characteristics determination did not exceed 3-5 %.
The membrane hydrogen-permeability was measured by chromatography at isothermal flow conditions. In the process of measurement we determined the hydrogen diffusion coefficients in the material of the membranes under study.
To conduct those measurements the samples of membrane under study were placed into a cell with design as shown below.
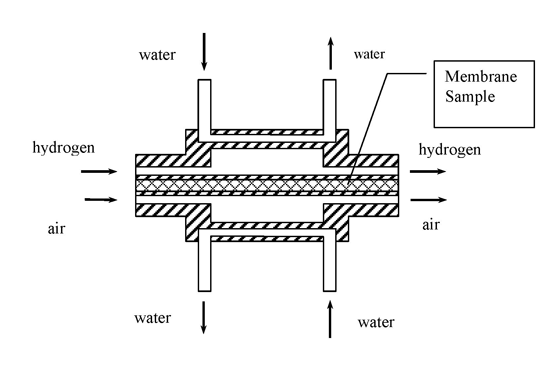
The cell for hydrogen permeability detection in the membrane samples
During those measurements the cell temperature varied while pressure remained unchanged and equal to atmospheric pressure.
Testing of membranes during water electrolysis was conducted in a cell with its membrane work surface 7cm2. Porous titanium plates were applied for its anode and cathode. Platinum black was used for the anode catalyst, and iridium black was used for the cathode catalyst. The electrolysis temperature was 80°C.
Membrane chemical resistance was determined by Fenton method keeping the membrane in 10% solution of hydrogen peroxide at 90°C during 30 minutes.
RESULTS AND DISCUSSION
Physico-chemical characteristics of the samples under study are shown in Table 2, from where one may see that humidity content is always higher for modified membranes. This increment is 3, 4, and 11% for Zr(HPO4)2, SPS, and PVA, correspondingly.
The insertion of polyvinyl alcohol into the perfluorinated membrane matrix, as one would expect, results in less Qvalues. Q-value growsif sulfated polysulfone with exchange capacity about 2 mmole/g is inserted into the membrane. However, the exchange capacity of membranes modified with Zr(HPO4)2 is significantly higher, testifying large amount of the inserted additive with ion-exchange properties.
Both humidity contents and exchange capacities were used to calculate the membrane specific humidity
content (nm, mole H2O/mole SO3-), that is the average number of
water moles per one mole of function groups. As one may see from Table 2, nm increases considerably
if the membrane is modified with polyvinyl alcohol because when so doing the sample humidity content
grows while its exchange capacity changes insignificantly. Hydrate capacity of membranes modified
with acid zirconium phosphate lessens by 2.6 times in connection with outrunning growth of its exchange
capacity as to compare with the growth of humidity content due to modification.
The results of measurements of concentration functions of specific electric conductivity for original and modified membranes in the solutions of muriatic acid and sodium chloride are presented in Fig.1 and 2. From those figures one may see that the addition of acid zirconium phosphate modifier results in the increase of perfluorinated membrane conductivity over all investigated concentration range for equilibrium solutions of HCl and NaCl. This is due to higher exchange capacity of hybride membranes and additional contribution of protons of acid zirconium phosphate to the total membrane conductivity. Similar increase in specific electric conductivity of HCl solutions is observed also for the membranes modified with sulfated polysulfone and polyvinyl alcohol, but here the effect is due to the growth of the electrolyte non-exchange sorption thanks to the membrane hydration that facilitates proton transport. However, in NaCl solutions the resistance of those samples exceeds that of original membranes bearing witness to the limited sodium ion diffusion mobility in such membranes.
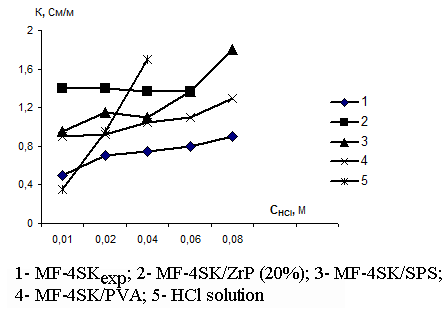
Fig. 1. The concentration dependence of membrane specific electric conductivity in muriatic acid solutions |
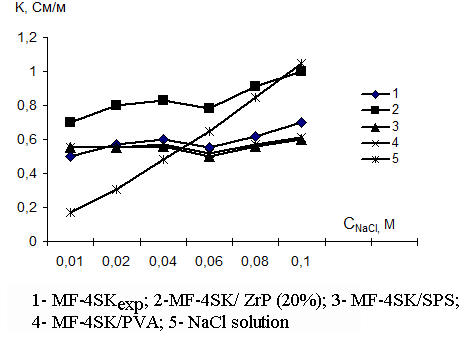
Fig. 2. The concentration dependence of membrane specific electric conductivity in sodium chloride solutions |
In Figs.3 and 4 there are the concentration dependencies of the integral diffusion permeability coefficients for original and modified membranes in HCl and NaCl solutions. From those figures one may see that diffusion permeability of membranes drops after the introduction of modifiers. In so doing the diffusion characteristics in HCl and NaCl solutions are very close because the diffusion process for electrolytes is limited by co-ions.
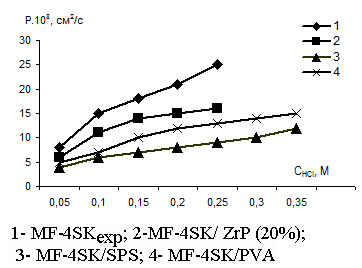
Fig. 3. The concentration dependence of the membrane diffusion permeability coefficients in muriatic acid solutions |
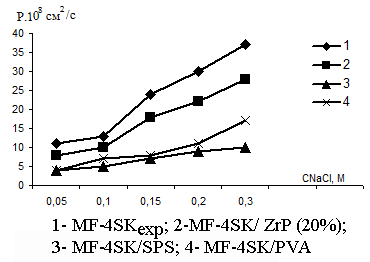
Fig. 4. The concentration dependence of the membrane diffusion permeability coefficients in sodium chloride solutions |
The voltammetric characteristics of the studied modified perfluorinated membranes are shown in Fig.5, the parameters of voltammetric curves are presented in Table 3.
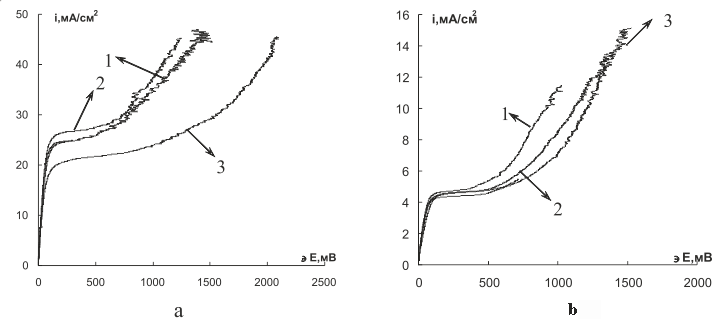
1 – original MF-4SK; 2 – MF-4SK/SPS; 3 – MF-4SK/ ZrP (20%)
Fig.5.Voltammetric characteristics of membranes in 0.05M HCl (a) and NaCl (b) solutions
Table 3. The parameters of membrane voltammetric characteristics in HCl and NaCl 0.05 М solutions.
|
Membrane |
ilimit,mA/cm2 |
ΔElimit, mV |
ΔEcr,mV |
Δ,mV |
|
0.05 M NaCl |
||||
|
MF-4CKorigin |
4.37±0.14 |
60.6±2.0 |
573.3±68.9 |
512.7±68.8 |
|
MF-4CK/SPS |
4.41±0.14 |
60.7±1.0 |
765.6±16.0 |
704.9±15.8 |
|
MF-4CK/ ZrP (20%) |
4.40±0.05 |
83.7±5.4 |
881.6±44.1 |
797.8±39.9 |
|
0,05 M HCl |
||||
|
MF-4CKorigin |
24.02±2.31 |
74.3±4.0 |
774.8±183.4 |
700.6±181.3 |
|
MF-4CK/SPS |
25.67±0.69 |
66.7±5.7 |
618.1±82.5 |
551.4±85.1 |
|
MF-4CK/ ZrP (20%) |
21.22±1.61 |
86.3±17.8 |
1329.4±114.8 |
1243.0±131.1 |
From those figures and table data we notice that for all samples their slopes of ohmic district of the polarization curves are nearly similar as the contribution of the membrane to the total resistance of the electro-membrane system is much less than that of the adjacent diffusion layers of the solution. The limit current value remains also unchanged within the range of the experimental error after the membrane modification. Some growth in size of the plateau of limit current independent of the modifier nature, and the large potential of the electric membrane system transition to its super limit state bear witness to the hindering of water decomposition in external electromagnetic field. This indirectly points to the retention of water by the membrane phase and confirms the performance of the modified samples.
The comparison of hydrogen permeability coefficients (Fig.6) indicates that the modification of membranes in all cases reduces them as to compare with those of original membranes; this undoubtedly must improve the purity of hydrogen generated by water electrolysis using solid polymer electrolytes. This effect is the most obvious with acid zirconium phosphate used for the modifier. And the difference between those coefficients even grows with temperature. Curve 6 in Fig.6 taken from [18] related to the study on Nafion-117 also confirms the said impact of modification.
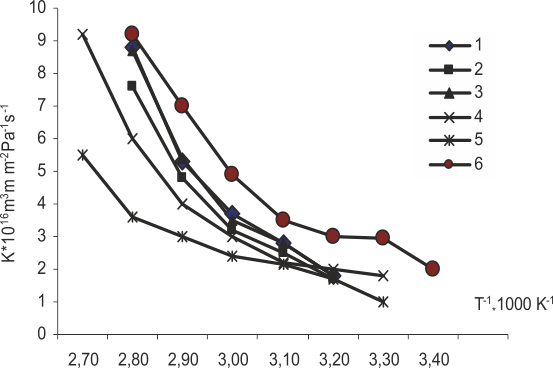
1- MF-4SKexp |
4-MF-4SKcast |
2- MF-4SK/SPS |
5-MF-4SK/ZrP (2,3%) |
3- MF-4SK/ZrP (20%) |
6- [18] |
Fig. 6. Dependence of the membrane gas permeability coefficient on the inverse temperature value
Testing of the membranes in the electrolyzer cell (Fig.7) lead to the conclusion that voltammetric characteristics of membranes modified with acid zirconium phosphate were practically similar to those of original membranes. Taking into account higher gross-hydration of those membranes (table2) that causes increasing of electro-osmotic transfer of water due to the proton migration one would expect better performance of electrolysers with anode water feeding. Higher voltage values when using PVA-modified membranes are connected most probably to the decrease in exchange capacity that governs mainly proton transfer.
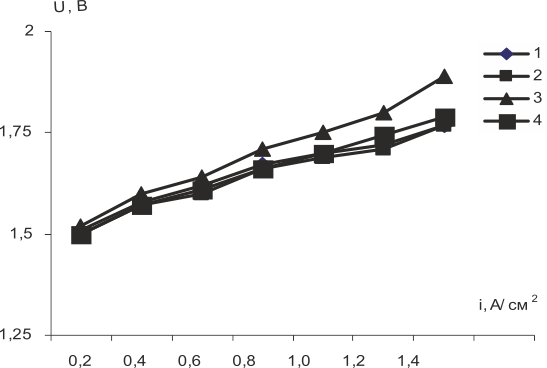
1- MF-4SKexp, 2-MF-4SKcast, 3- MF-4SK/PVA, 4- MF-4SK/ZrP (20%)
Fig.7. Voltammetric characteristics of the cell during water electrolysis with membranes
In order to estimate the membrane resources they were tested by the Fenton method (Table 4). Fluoride-ion emission per 1 g of dry membrane, specific volume electric resistance (ρV) and appearance were marked prior and after testing.
Table 5. The properties of membrane treated with Fenton solution
|
# |
Membrane |
Emission of fluoride-ion CF, mg-eqv/g |
ρV, Ohm*cm |
Appearance |
|
|
Prior to test |
After test |
||||
|
1 |
Nafion-117 |
0.0028 |
10.5 |
10.6 |
unchanged |
|
3 |
MF-4SKexp |
0.0022 |
10.3 |
10.5 |
unchanged |
|
4 |
MF-4SKcast |
0.0025 |
9.0 |
8.8 |
unchanged |
|
5 |
MF-4SK/ ZrP (20%) |
0.0024 |
9.2 |
9.3 |
unchanged |
|
7 |
MF-4SK/PVA |
- |
- |
- |
Bubbling of |
From Table 5 one may see that membranes with acid zirconium phosphate remain unchanged while those modified with PVA display bubbles generating within the membrane, possibly due to PVA destruction in contact with hydrogen peroxide. The data on Nafion-117 membrane are shown for comparison.
Conclusions
The analysis of the results of complex study on electric transport and gas diffusion characteristics of perfluorinated membranes modified with organic and inorganic substances allows choosing of the most promising approach to its application in various electrochemical units. It is established that the introduction of modifiers neither decrease the membrane electric conductivity, nor affect considerably its voltammetric parameters characteristics, however, it diminishes significantly its diffusion permeability, by hydrogen, first of all, and increases electro-osmotic transfer of water. However, the membranes modified with acid zirconium phosphate with their higher chemical stability are more promising for using in membrane electrolyzers.
Literature
- Kuleshov N.V., Fateyev V. N,, Grigoriev S.A. Working of electrochemical systems with solid polymer electrolyte //High technologies. - 2004, N 10 p.85-89
- S.A. Grigoriev, P. Millet, V.N.Fatev Evolution of carbon-supported Pt and Pd nanoparticles for the hydrogen evolution reaction in PEM water electrolysers // J. of Power Sources, 2008, vol. 177, issue 2, p. 281-285
- Kreuer K.D. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells, J. Membr Sci.– 2001. –V.185. – P. 29-39.
- Sata T., Sata, T., Yang W. Studies on cation-exchange membranes having permselectivity between cations in electrodialysis // J. of Membrane Sci. – 2002. – Vol. 206. – P. 31-60.
- Nagarale. R.K., Gohil G.S., Shahi V.K. Recent developments on ion-exchange membranes and electro-membrane processes / R.K. Nagarale, // Advances in Colloid and Interface Sci. – 2006. – Vol. 119. – P. 97 – 130
- Jaroslavtsev A.B., Zabolotsky V. I., Nikonenko V.V. Ion transfer in membrane and iono-exchange materials//Successes of chemistry. - 2003. - 72, N 5. – p/ 438-470.
- Dobrovolskij Ju.A., Dgannash P., Laffit B, Belomoina N.M., Rusanov A.L., Lichachev D.Ju. Successes in area protonic polymerelectrolytic membranes// Electrochemistry . 2007. - 43. - N 5. – p. 515-527.
- Volkov V.V., Mchedlishvili B.V., Roldugin V. I., Ivanchev S.S., Jaroslavtsev A.B.//Russia nanotechnologies. 2008. 3. N 11. p. 21-53
- Yeo R.S. Applications of perfluorosulfoneted polymer membranes in fuel cells, electrolyzers, and load leveling devices // Perfluorinated Ionomer Membranes/ Eds. A. Eisenberg, Y.L/ Yeager/ ACS Symposium Series/ 1982, Vol. 180, p. 453-474
- Panshin Ju.A., Drejman N.A., Andreeva A.I., Manechkina O. N. Properties of perfluorosulfocathionitic membranes MF-4SK//Plastics. 1977, N 8, p. 7-8
- Patent RF № 2267498 Linear statistical terpolymer of the tetraftoretylene with functional perfluorocomonomers and a methode of its getting //Bobrova L.P., Ostrizhko F.N., Timofeev S.V., Derjuzhov J.M.//Inventions. Useful models. 2006
- Bauer F., Willert-Porada M. Microstructural characterization of Zr-phosphate-Nafion membranes for direct methanol fuer cell (DMFC) applications // J. of Membrane Sci. – 2004. – Vol. 233 – P.141-149.
- Daiko Yu., Klein L.C., Kasuga T., Nogami M. Hygroscopic-oxides/Nafion- hybrid electrolyte for direct methanol fuel cells // J. of Membrane Sci. – 2006. – Vol. 281 – P.619-625.
- Gnusin N.P., Berezina N.P., Dyomina O. A, Kononenko N.A. Physicochemical principles of testing of the ionexchange membranes//Electrochemistry. - 1996. 32, N 2. - p.173-182.
- Berezina N.P., Kononenko N.A., Dyomina O.A., Gnusin N.P. Characterization of ion-exchange membrane materials: properties vs structure //Advances Colloid Interface Sci. 2008. – V.139. – P.3-28.
- Loza N.V., Kononenko N.A., Shkirskaja S.A., Berezina N.P. Polarization characteristics of the ionexchange membranesMF-4SK depending on a method of their modifying// Electrochemistry. - 2006. - 42, N 8. - p.907-915.
- Berezina N.P., Kononenko N.A., Loza N.V., Sycheva A.A.-P. Research of the electrochemical behaviour of composites on the basis of MF-4SK and polyaniline with a method of membrane voltamperometry//Electrochemistry. 2007. - 43. - N 12. - p. 1417-1427.
- Broka K, Ekdunge P. Oxygen and hydrogen permeation properties and water uptake of Nafion 117 membrane and recast film for PEM fuel cell // J. Appl. Electrochem. 1997, 27, р. 117–123
Recommended for publication by V.V. Kornilov
Fluorine Notes, 2011, 76, 7-8
