Received: April, 2011
Fluorine Notes, 2011, 76, 1-2
New reaction of polyfluorinated alcohols with thionyl chloride
A.I. Rakhimov and A.V. Miroshnichenko
Volgograd State Technical University, Russia, 400131, Volgograd, Lenin Prosp., 28
e-mail: organic@vstu.ru
Abstract: Reaction between polyfluorinated alcohols H(CF2CF2)nCH2OH, where n = 1 – 4, and thionyl chloride taken in equimolar ratio occurs in the presence of catalytic amounts of N,N-dimethylformamide to form dipolyfluoroalkyl ethers in yields 54.7 to 96.4%.
Keywords:Polyfluorinated alcohol, N,N-dimethylformamide, thionyl chloride, dipolyfluoroalkyl ether
It is well known [1-3] that polyfluorinated alcohols (PFA) react with an excess amount of thionyl chloride in the presence of catalytic amounts of N,N-dimethylformamide (DMF) to give polyfluoroalkyl chlorosulphites (PFACS). Polyfluoroalkyl chlorosulphites were used in the reaction with alcohols for the synthesis of polyfluoroalkyl ethers [4-9].
In this work we showed that dipolyfluoroalkyl ethers can be formed directly in catalytic (DMF) reaction of PFA with thionyl chloride.
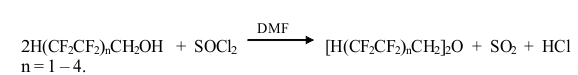
It was established that the yield of symmetrical dipolyfluoroalkyl ether depends on the ratio of the reactants. The yield is maximum when the molar ratio PFA : thionyl chloride : DMF is 1 : 1, the DMF amount is 2-3-fold higher than in the case of synthesizing polyfluoroalkyl chlorosulphites, i.e. it is 0.005-0.01 mole per 1 mole PFA.
Taking into account the PFA acidity and their ability to give associates with proton acceptors [10], it is obvious that proton-donor complexes between PFA and thionyl chloride as well as associates involving DMF are formed in the reaction medium.
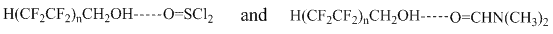
Possibly, formation of complexes favors the one-step reaction.
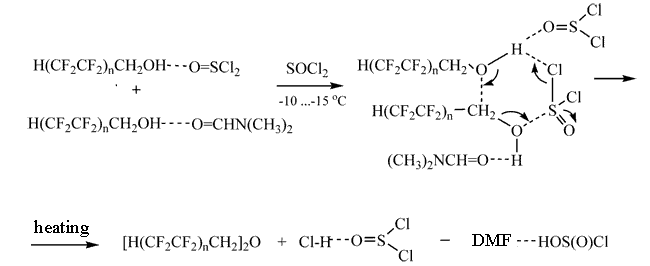
In the six-membered polar transition state the proton transfer occurs from one of PFA molecules to the polar chlorine in the thionyl chloride molecule, and the nucleophilic attack of the oxygen of the polyfluoroalkoxy group to the positively charged carbon of the other molecule of PFA included in the six-membered cycle. The hydroxyl group transfers simultaneously from the carbon to the more electron-acceptor sulfur. Unstable chlorosulphinic acid easily decomposes to give hydrogen chloride and sulfur dioxide [11].
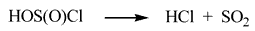
The following yields of dipolyfluoroalkyl ethers were observed in the one-step synthesis for the series of PFA.
|
PFA, n |
Yield of ether, % |
|
1 |
75.8 |
|
2 |
96.4 |
|
3 |
77.3 |
|
4 |
54.7 |
In the case of the synthesis of the same ethers using the two-step method [8], the following yields of dipolyfluoroalkyl ethers were observed.
|
PFA, n |
Yield of PFACS (the 1st step), % |
Yield of ether |
Yield of ether on a basis of taken PFA, % |
|
1 |
76.7 |
98.5 |
75.5 |
|
2 |
88.5 |
59.0 |
52.2 |
|
3 |
79.4 |
57.3 |
45.5 |
|
4 |
31.0 |
The compound has not been obtained in the two-step synthesis |
Thus the one-step method has the evident advantages consisting in the higher yield and the lower laboriousness (steps of isolation and purification of PFACS are absent). The constants of the synthesized dipolyfluoroalkyl ethers are given below.
|
Ether [H(CF2CF2)nCH2]2O |
n |
bp, °C / mm Hg (mp, °C) |
d204, |
nD20 |
|
Di(1,1,3-trihydroperfluoropropyl)ether |
1 |
65/1 |
1.6251 |
1.3575 |
|
Di(1,1,5-trihydroperfluoropentyl)ether |
2 |
103/2 |
1.7344 |
1.3385 |
|
Di(1,1,7-trihydroperfluoroheptyl)ether |
3 |
130/1 |
1.8014 |
1.3370 |
|
Di(1,1,9-trihydroperfluorononyl)ether |
4 |
(47–48) |
- |
- |
As we can see the ether density grows from 1.6251 to 1.8014 g/cm3 as the perfluorinated chain length increases.
Experimental
The structure of the compounds obtained was confirmed by IR and 1H NMR spectroscopy. The IR spectra were recorded on a Spekord – M8 instrument, in thin layer (liquid films) and chloroform solution. 1H NMR spectra were recorded on a Mercury-300 (Varian) spectrometer operating at 300 MHz with tetramethylsilane used as internal standard.
Synthesis of di(1,1,3-trihydroperfluoropropyl) ether [HCF2CF2CH2]2O. Typical procedure
A mixture of 1,1,3-trihydroperfluoropropanol (15.8 g, 0.1197 mole) and DMF (0.043 g, 0.0006 mole) was added to thionyl chloride (14.2 g, 0.1197 mol) at –15°C with vigorous stirring and blowing with dry nitrogen for 30 min, then the temperature was increased to 20°C and stood for 1 h; afterwards the temperature was increased to 30 – 40°C and stood for additional 4 h. An excess of thionyl chloride (bp 79 ºC), unreacted 1,1,3-trihydroperfluoropropanol (bp 109°C), and byproduct – chlorosulphite – (bp 36°C /5 mm Hg) were distilled off successively. Distillation of the residue gave 11.16 g (75.8%) of di(1,1,3-trihydroperfluoropropyl) ether, mp 65ºC (1 mm Hg), nD20 1.3575, d204 1.6251. IR (ν, cm–1): 1111 s (νC-O-C); 1223 s (νCF2); 2850 m, 2920m, 2960m (ν CH2); 3008w (CHF2). 1H NMR (δ, ppm): (SSCC, J, Hz): 5.803 tt (52.8, 3.3) (2H, HCF2); 4.274 q (13.2) (4H, CH 2).
Therefore, a new method for the preparation of symmetrical dipolyfluoroalkyl ethers has been developed using reaction between polyfluorinated alcohols and thionyl chloride in the presence of DMF as a catalyst at the reactant molar ratio (PFA : thionyl chloride : DMF) equal to 1 : (1 – 1.1) : (0.005 – 0.108). This method makes it possible to obtain the ethers in yields 54.7 to 96.4% directly from polyfluorinated alcohols and thionyl chloride deleting the step of isolation and purification of polyfluoroalkyl chlorosulphites, this significantly simplifies the process of preparation of the ethers.
Literature
- Rakhimov A.I., Nalesnaya A.V., Fedunov R.G. and Vostrikova O.V. Zh. Obshch. Khim., 2004, vol. 74, N5, p. 868.
- Rakhimov A.I, Nalesnaya A.V., Vostrikova O.V. Pat. 2247110 Russia, 7 C 07 C 301/00, 2005.
- Rakhimov A.I., Nalesnaya A.V. Fluorine compounds. Chemistry. Technology, Application . St.-Petersburg: Ross.Nauch. Tsentr. 2009, p. 288-296.
- Rakhimov A.I., Fisechko R.V. Zh. Obshch. Khim. 2007. vol. 77, N 10, p. 1750-1751.
- Rakhimov A.I., Fisechko R.V. Zh. Obshch. Khim. 2008. vol.. 78, N 2,p. 338.
- Rakhimov A.I, Nalesnaya A.V., Vostrikova O.V. Zh. Obshch. Khim. 2008, 78, N 11. p. 1842-1848.
- Rakhimov A.I, Nalesnaya A.V., VostrikovaO.V. Zh.Org.Chem. 2004, 74. ,N 4, p. 967.
- Rakhimov A.I, Nalesnaya A.V . Pat. 2312097 Russia, С 07 С 43/12, 41/01. 2007.
- Rakhimov A.I, Nalesnaya A.V., Vostrikova O.V. Zh.Prikl. Chem. 2004, 77, N 9, p.1573 – 1574.
- Rakhimov A.I, Nalesnaya A.V., Vostrikova O.V., Storozhakova N.A. Chemistry and Technology of elementorganic monomers and polimeric materials. Volgograd 2004, N 2, p. 39-41.
- Andersen К. К. Sulfinic acides. Obshch. organic Chemistry, v.5. M. : Chemistry 1983,p. 497.
Recommended for publication by Prof. Alexander I. Rakhimov
Fluorine Notes, 2011, 76, 1-2
