Fluorine Notes, 2002, 21, 3-4
Ozone relevant chladones in Russia
Prof., Doctor of Chemical science B.N.Maximov
RSC "Applied Chemistry" 14, Dobrolubov ave., St.-Petersburg, 197198, Russia
As is well known, the Russian Federation as the assignee of the USSR, ratified in 1988y. the Montreal protocol on substances that deplete the ozone layer, completely acknowledged and undertook the obligations of the Montreal agreement to phase out production and consumption of ozone depleting substances that was already fulfilled in the middle of 2000y.
The main work on development of technology of manufacture of ozone relevant chladones (freons) by decrees of the Russian Federation Government was commissioned to Russian Scientific Center "Applied Chemistry", a leading enterprise in the country on technology of fluorine compounds.
This paper presents main results of this work that made possible to solve this problem in Russia. The work was awarded by the prize of the Russian Federation Government in 2001y.
Chladones as chemical substances possess a number of unique properties: they are chemically inert, explosion and fire proof, nontoxic. Due to that they have found wide application in technique as refrigerants, solvents, fire-extinguishers, frothers in plastic industry etc.
But destructive effect of some chemical substances including bromine and chlorine containing ones on the ozone layer found in the 80 yy. attracted attention to a big group of industrial chladones.
International documents on protection of the ozone layer were the Vienna Convention of 1985 and then the Montreal Protocol of 1987 on substances that deplete the ozone layer.
According to the Montreal Protocol a list of ozone depleting substances was determined including chlorofluorocarbons, bromofluorocarbons (halons) and some chlorocarbons. The list of main ozone depleting substances under the Montreal Protocol is given in table1.
Table1. Ozone depleting substances controlled by the Montreal protocol
| Group of chemical compounds |
Chladone name |
Ozone depletion potential of chemical compounds (ODP)* |
| Grooup I | ||
| CFCl3 | R-11 | 1,0 |
| CF2Cl2 | R-12 | 1,0 |
| CF2Cl-CFCl2 | R-113 | 0,8 |
| CF2Cl-CF2Cl | R-114 | 1,0 |
| CF3-CF2Cl | R-115 | 0,6 |
| Group II | ||
| CF2ClBr | Halon 1211 | 3,0 |
| CF3Br | Halon 1301 | 10,0 |
| CF2Br-CF2Br | Halon 2402 | 6,0 |
ODP of R-11 was taken as equal to 1.
Later the list of the controlled compounds was considerably expanded for tetrachloromethane (ODP=1.1), chloroform (ODP=0.1) and more than 70 chloro-, bromohydrocarbons of different structure of methane, ethane and propane series.
The Montreal Protocol has obliged countries accepted it to limit their import, export, consumption and production of the ozone depleting substances.
For developed countries the period of phase out production and consumption expired in 1996, for developing countries it was prolonged to 2015, for the Russian Federation it expired in the middle of 2000y.
The Russian Federation was one of the largest world manufacturer of chladones, ozone depleting substances. In 1987y its share of the world production was equal to 15%, almost all industrial branches were consumers of chladones.
Thus, the refrigeration equipment sector includes 11 plants -manufacturers of house hold equipment, 5 plants-manufacturers of trade equipment, 5 manufacturers of industrial equipment and hundreds enterprises go into service.
A lot of enterprises are in manufacture of foam plastics, aerosol medicines, fire-extinguishers, a great number of aviation, shipbuilding plants.
As a result of investigation a nomenclature of ozone relevant chladones was determined and presented in table 2.
Table 2. Nomenclature of ozone relevant chladones and their mixtures.
| Compounds |
Chemical Formula |
ODP |
Combustibility |
ToxicityATL (TLV) (ррm) |
|
Chladon 134a |
CF3-CFH2 |
0 |
no |
1000 |
|
Chladon 152a |
CF2H-CH3 |
0 |
yes. |
1000 |
|
Chladon 125 |
CF3CF2H |
0 |
no |
1000 |
|
Chladon 143a |
CF3-CH3 |
0 |
yes. |
1000 |
|
Chladon 32 |
CF2H2 |
0 |
yes. |
1000 |
|
Chladon 218 |
CF3-CF2-CF3 |
0 |
no |
1000 |
|
Chladon C318 |
C4F10 - c |
0 |
no |
1000 |
|
Chladon 227ea |
CF3-CFH-CF3 |
0 |
no |
1000 |
|
Chladon 23 |
CF3H |
0 |
no |
1000 |
|
Chladon 22 |
CF2ClH |
0,05 |
no |
1000 |
| Chladon 142b | CF2Cl-CH3 | 0,05 | yes. | 1000 |
|
Chladon 141b |
CFCl2-CH3 |
0,05 |
yes |
1000 |
|
C10M1 |
Chladon 22/ Chladon 21/ Chladon 142b |
0,05 |
no |
1000 |
|
C10M2 |
Chladon 22/ Chladon 21/ Chladon 134а |
0,05 |
no |
1000 |
|
C1 |
Chladon 152а/iso-butane |
0 |
yes |
1000 |
|
Blend |
Chladon 125/ Chladon 227еа/ iso-butane |
0 |
no |
1000 |
|
Blend |
Chladon 125/Chladon 134а/iso-butane |
0 |
no |
1000 |
|
Blend |
Chladon 125/ Chladon 32/ iso-butane |
0 |
no |
1000 |
According to the conclusions of the Montreal Protocol Commission and taken into account a wide application in technique of some chlorine-containing chladones ( for example, chladones 22, 142b) and their low ODP (0.05-0.06) and also difficulties in their replacement in a short space of time, it was decided to use them up to 2030y.
In the development of technologies of manufacturing new chladones there were used mainly two methods of their production in dependence on their structure: methods of gas phase and liquid phase fluorination on catalysts (see scheme1).
Scheme1. Methods of new chladons manufacturing
1. Gas phase fluorination by anhydrous hydrogen fluoride of corresponding chloroorganic compounds on catalysts (134a, 125 and others)
2. Liquid phase fluorination by anhydrous hydrogen fluoride of chloroorganic compounds (152a, 32, 22, 141b, 142b, 143a)

A brief description of synthesis of some chladones is given below.
Chladone 134a
On the basis of technical and economic evaluation and taken into account sources of raw materials, a method of hydrofluorination of trichloroethylene was chosen for development of technology to produce chladone 134a at a temperature of 350-400oC, pressure of 0.4-0.8 MPa on chromomagnesium fluoride catalyst. The process of chladone 134a synthesis may be divided into two main stages:
1. hydrofluorination of trichloroethylene to 1,1,1-trichloroethane (chladone 133a)
CHCl=CCl2 + 3HF  CF3-CH2Cl + 2 HCl + 93 kJ/mol
CF3-CH2Cl + 2 HCl + 93 kJ/mol
2. hydrofluorination of chladone 133a to chladone 134a:
CF3-CH2Cl + HF 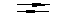 CF3CFH2 + HCl -18 kJ/mol
CF3CFH2 + HCl -18 kJ/mol
The first process stage is practically irreversible and the content of chladone 133a in the organic part of the synthesis products is 90-98% by volume.
The second stage is of reversible character and the content of chladone 134a in the organic part of the synthesis products is 20-40%.
Due to reversibility of the second process stage it is necessary to design the reactor unit in such a way that to remove hydrogen chloride from the reaction zone and to return back high boiling synthesis products (chladone 133a) to the synthesis unit by recycle. Chladone 134a produced in such a way contains 99.9% of the main substance.
Chladone 125.
For synthesis of chladone 125 a method of hydrofluorination of tetrachloroethylene by anhydrous hydrogen fluoride on chromomagnesium fluoride catalyst was used. The study of the process and its thermodynamic analysis have shown that the process may be presented of two main stages:
1. hydrofluorination of tetrachloroethylene to 1,1,1,2-tetrachlorofluoroethane (chladone 124)
CCl2=CCl2 + 4HF CF3-CFClH + 3HCl + 82 kJ/mol
CF3-CFClH + 3HCl + 82 kJ/mol
2. hydrofluorination of chladone 124 to chladone 125:
CF3-CFClH + HF  CF3-CF2H + HCl - 7,89 kJ/mol
CF3-CF2H + HCl - 7,89 kJ/mol
Selectivity of chladone 125 exceeds 90%, a method of purification by extractive fractionation was developed for isolation of chladone 125.
Chladone 227ea.
Chladone 227ea is an effective fire extinguishing agent. The process of its manufacture is fulfilled according to the following scheme:
CF3-CF=CF2 + HF  CF3-CHF-CF3 + 166 kJ/mol
CF3-CHF-CF3 + 166 kJ/mol
The reaction may have place both in gas phase and in liquid phase, but the liquid phase process requires expensive catalysts (based on Ta, Nb) and a considerable time of being in the reaction zone.
Due to that for industrial implement a process of gas phase catalytic hydrofluorination of hexafluoropropylene on chromomagnesium fluoride catalyst was chosen.
The process of hexafluoropropylene hydrofluorination runs most effectively at a temperature of 350-450oC and at the mole ratio of components HF:CF3-CF=CF2 as 1-5:1, the content of chladone 227ea in the reaction phase is 50-70% by volume at selectivity of 97-98%.
Chladone 23.
A method of producing chladone 23 is based on disproportionation of chladone 22 on chromomagnesium fluoride catalyst or on activated aluminium oxide:
An optimal temperature for the synthesis of chladone 23 is a temperature of 250-350oC.
Chladone 32.
Chladone 32 is a component of low temperature refrigerant blends. A method of its production is based on liquid phase fluorination of methylene chloride at a temperature of 95-105oC and pressure of 1.5-2 MPa in the presence of catalyst, antimony pentachloride, the following reactions take place in this case:
CatF, CatCl are fluorinated and chlorinated forms of the catalyst accordingly.
The selectivity of chladone 32 in the process is 99%, the content of the main substance is 99.9%.
Chladone 152a.
A process to produce chladone 152a is based on liquid phase hydrofluorination of vinyl chloride at a temperature of 90oC and pressure of 0.6-0.8 MPa in the presence of tin tetrachloride as the catalyst.
The process includes few stages, it is easy in equipment implementation, has a high output and provides a long life time of the catalyst.
Chladones 141b, 142b,143a.
The most economical method of synthesis of chladones 141b, 142b, 143b is a method of hydrofluorination of vinylidene chloride without catalyst.
The process runs at a temperature of 90-110oC, pressure of 0.6-1.-MPa without resinification of the starting vinylidene chloride.
The foregoing processes of manufacturing chladones have been patented by the Russian federation patents. The production of ozone relevant chladones implemented or is establishing at the following industrial plants of Russia: JSC "Halogen" ( Perm-city), JSC "Kaustik" (Volgograd), JSC "Chimprom" (Volgograd), JSC "Kirovo-chepetsky Chemical Plant" (Kirovo-Chepetsk), JSC "Redkinsky Plant" (Redkino), RSC "Applied chemistry". At RSC "Applied Chemistry" there are plants on recovery of chladones and halons.
The works connected with development of refrigerant blends containing several chladones ( including hydrocarbons) which most completely comply with the properties of the prohibited refrigerants (chladone 12 etc.) take an important place in the development of ozone relevant refrigerants.
According to the Montreal Protocol the chladones with zero ODP and providing nonflammable compositions are used as ozone relevant components for refrigerant blends ( chladones 134a, 125, 143a, 32,23,152a,227ea,218,318c).
A number of mixtures (table 2) were developed for service maintenance, they contain transition chladones (ODP=0.05) which properties are close to freon12 replaced. An important condition is compatibility of the new refrigerant blends with compression oils of domestic manufacture.
The new refrigerant blends have been patented in the Russian Federation.
The new refrigerant mixtures has been tested in house hold refrigerators, refrigeration chambers of a large volume, it was shown that the new refrigerants provided maintenance of the technical characteristics close to the characteristics of the use of chladone 12.
References
1. Maksimov B.N., Barabanov V.G.‚ Zotikov V.S. i dr. Promyshlennye ftororganicheskie produkty, Sprav. izd. S.Pb‚ Khimiya, 1996 (544 s.)
2. Barabanov V.G‚ Zotikov V.S. Maksimov B.N. i dr, Galogensoderzhashchie pozharotushashchie agenty. S.-Peterburg‚ TEZA, 1999 (130 s.)
3. Barabanov V.G.‚ Maksimov B.N., Kontseptsiya perevoda promyshlennosti Rossii na ozonobezopasnye veshchestva. Tezisy dokladov 1j Mezhdunarodnoj konferentsii "Khimiya, tekhnologiya i primenenie ftorsoderzhashchih soedinenij“, S.Peterburg‚ 1994 g.
4. Maksimov B.N. Osnovnye tendentsii razvitiya promyshlennosti ftorproduktov. Tezisy dokladov 2j Mezhdunarodnoj konferentsii “Khimiya, tekhnologiya i primenenie ftorsoderzhashchih soedinenij”. S.Peterburg‚ 1997 g.
5. Patents RU:
№ 1794308‚ 1990.
№ 1825485, 1992.
№ 2052442, 1992.
№ 2039032. 1993.
№ 2051140, 1993.
№ 2049085, 1993.
№ 2065430, 1994.
№ 2051139, 1995.
№ 2088626, 1991.
№ 2124493, 1997.
№ 2098445, 1997.
№ 2117025‚ 1997.
№ 2141467‚ 1997.
№ 2135541. 1998.
№ 2161637‚ 1999.
Fluorine Notes, 2002, 21, 3-4
