Fluorine Notes, 2002, 21, 1-2
Use of hydrogen fluoride and its complexes with bases for introduction of fluorine atoms into organic molecules
G.G.Furin
Novosibirsk Institute of organic chemistry named after N.N.Vorozhtsov Siberian branch of the Academy of Science of Russian Federataion
Fax: +7-3822-344752
e-mail: furin@nioch.nsc.ru
Annotation
The review summarizes and systematizes up-to-date data on fluorinating ability of anhydrous hydrogen fluoride and its complexes with bases of unsaturated organic compounds, alcohols, diazoketones, hydrazones and oximes of ketones, 3,3-dialkyl-1-aryltriazenes, aryldiazosulfides etc.. It contains an analysis of main achievements in use of anhydrous hydrogen fluoride as a fluorinating agent to produce ozone-friendly freons in gas and liquid phases both without catalysts and in the presence of latter. There has been examined factors influencing opening three-membered cycles containing oxygen and nitrogen atoms. The review contains examples of practical application of different groups of fluoroorganic compounds, rational methods of their production and their role in development of modern industry .
Contents
Introduction. Hydrogen fluoride as a basic stock substance in chemical industry.
1. Fluorination with anhydrous hydrogen fluoride and its complexes with bases of compounds from different classes.
1.1.Hydrofluorination of unsaturated compounds
1.1.1. Influence of anhydrous hydrogen fluoride on
unsaturated compounds
1.1.2. Hydrofluorination of unsaturated compounds by hydrogen fluoride
complexes with bases
1.1.3. Reactions of anhydrous hydrogen fluoride and its complexes containing
bases with acetylene derivatives
1.1.4. Fluorination of alkenes with hydrogen fluoride in the
presence of catalysts
1.1.5. Fluorination of unsaturated compounds with hydrogen fluoride in the presence of
electrophilic reagents
2.Processes of replacement of functional groups with fluorine atoms.
2.1. Replacement of oxy-group with fluorine under effect of hydrogen fluoride complexes containing bases.
2.2. Reactions with hydroxylamines, hydrazones and oximes of ketones.
2.3. Reactions with diazoketones,
3,3-dialkyl-1-aryltriazenes and aryldiazosulfides.
2.4. Exchange reactions of haloids under effect of hydrogen fluoride in the presence of catalysts
3. Opening nitrogen- and oxygen-containing three-membered heterocycles
3.1.Opening an epoxy ring by anhydrous hydrogen fluoride and its complexes with bases.
3.2. Opening
nitrogen-containing three-membered heterocycles.
Conclusion.
1.1.5.Fluorination of unsaturated compounds by hydrogen fluoride in the presence of electrophilic reagents.
Addition of a positive particle X+ (Cl+, Br+, I+) of a halogen in the presence of a fluoride-ion source to form a product of trans-addition of X-F elements to the double bond of the olefin is a selective reaction for a wide range of unsaturated compounds. The source of X+ cation is mainly such reagents as halogen-amides (N-bromosuccinamide, N-bromoacetamide etc.) or free halogen (I2 or Br2). The fluoride-ion source is usually anhydrous hydrogen fluoride or its complexes with bases.
Above there were mentioned examples of the use of anhydrous hydrogen fluoride and its complexes for implementation of the hydrofluorination process. The latter runs in two stages: at first hydrogen fluoride as an acid protonates an unsaturated organic compound or a substrate containing a heteroatom with unpaired electron couple to generate a cationoid particle. Further an attack of fluoride ion towards the carbon atom with the greatest positive charge takes place. This process scheme may be realized otherwise. At first due to interaction of electrophilic agents with the multiple bond or due to a different influence on the bond a carbanion is generated and hydrogen fluoride or its complex with a base then becomes the fluoride-ion source. Introduction of fluorine into the organic molecule takes place in this case also but a different functional group is involved. It should be noted that this approach was found very productive and was widely used. This review presents only examples illustrating opportunities of using hydrogen fluoride and its complexes with bases in such a way.
Generally the reaction runs according to the following scheme. At first the cationoid particle reacts
with the multiple bond to form halogenoid cation which is subjected to the attack of the fluoride-ion
to give, as a rule, a product of anti-addition . So, in case of interaction of N-chlorosuccinamide
in a medium of anhydrous hydrogen fluoride with alkenes there is formed 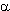 -chloro-
-chloro-
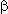 -fluoroalkane.
-fluoroalkane.
AgF, BF3 etc. are often used in these processes as catalysts. Et3N/3HF system is an universal source of fluoride-ion in reactions of halogenfluorination of unsaturated hydrocarbons [168]. Table 10 presents the examples of stereo- and regio-selective synthesis of organic compounds by chlorofluorination and bromo-fluorination of alkenes.
Table 10. Halogenfluorination of alkenes
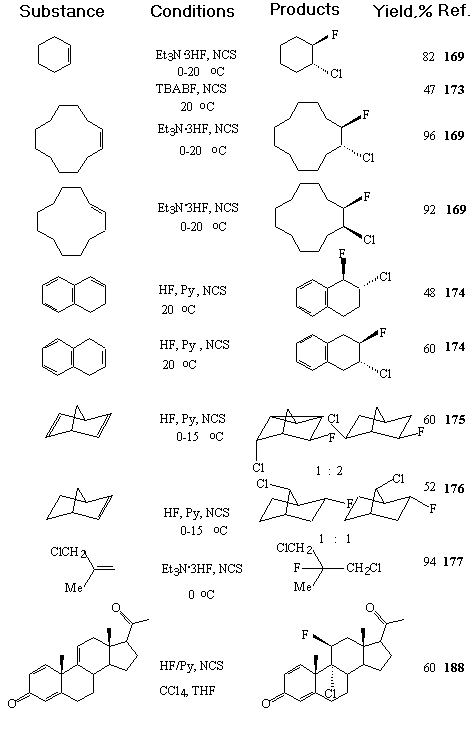 |
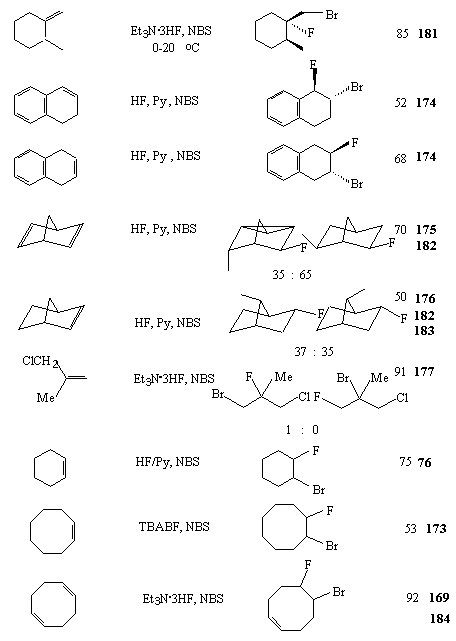 |
| Substance | Conditions | Products | Yield,% | References |
| CH2=CH2 | HF/Py, NBS | CH2FCH2Br |
30 |
179 |
| MeCH=CH2 | HF/Py, NBS | MeCHFCH2Br |
40 |
179 |
| Me2C=CH2 | HF/Py, NBS | Me2CFCH2Br | 85 | 179 |
| BuCH=CH2 | HF/Py, NBS | BuCHFCH2Br | 90 | 179 |
| (Z)MeCH=CHMe | HF/Py, NBS | MeCHFCHBrMe | 46 | 180 |
| (Z)EtCH=CHEt | HF/Py, NBS | EtCHFCHBrEt | 58 | 180 |
| PhCH=CH2 | HF/Py, NBS | PhCHFCH2Br | 53 | 185 |
| BnCH=CH2 | HF/Py, NBS | BnCHFCH2Br | 74 | 186 |
| Ph2C=CH2 | HF/Py, NBS | Ph2CFCH2Br | 78 | 182,187 |
| Ph2C=CHMe | HF/Py, NBS | Ph2CFCHBrMe | 68 | 185 |
| MePhC=CH2 | HF/Py, NBS | PhCMeFCH2Br | 72 | 185 |
| MePhC=CH2 | Et3N*3HF, NBS | PhCMeFCH2Br | 97 | 185 |
| ClCH=CMeCOOMe | HF, NBS | CHClFCBrMeCOOMe | 57 | 186 |
| ClCH=CFCOOMe | HF, NBS | CHFClCBrFCOOMe | 39 | 186 |
| ClCH=CClCOOMe | HF, NBS | CHFClCBrClCOOMe | 41 | 186 |
| PhCCl=CHCOOEt | HF/Py, NBS | PhCClFCHBrCOOEt | 188 | |
| BzCH=CHPh | HF/Py, NBS | BzCHBrCHFPh | 40 | 189 |
A combination of Et3N/3HF system and N-halosuccinimides was found an effective halofluorinating reagent of alkenes [169,170]. The reaction runs at room temperature in glassware. The anti-addition has a stereospecific character.
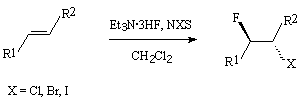
It has been shown [171] that the fluorinating agent being a complex of trialkylamine/HF ( for example Me3N/3HF in combination with N-bromosuccinimide (NBS)) in reactions with styrenes is more effective in comparison with Et3N/3HF and gives bromofluorination products according to Markovnikov law with high regioselectivity.
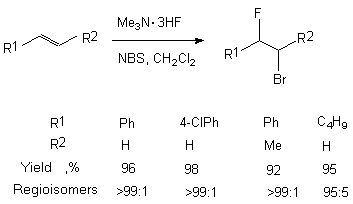
In case of terminal aliphatic alkenes, hexene for example, the regioselectivity decreases a little at transition from Me3N/ 3HF (95:5) system to Et3N/3HF (87:13) system.
The high regioselectivity takes place in bromofluorination of terminal olefins when Et3N 3HF complex is used in the presence of N-bromosuccinimide [172] The yield of the products is almost quantitative.
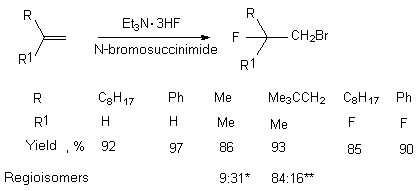
- 1-bromo-2-fluoro-3,3-dimethylbutane: 2-bromo-1-fluoro-3,3-dimethylbutane
- 1-bromo-2-fluoro-2,4,4-trimethylpentane: 2-bromo-1-fluoro-2,4,4-trimethylpentane
In case of cis- and trans-6-dodecene there are formed threo- and erythro-6-bromo-7-fluorododecenes respectively in a quantitative yield. (E)-6-bromo-7-fluoro-3,7-dimethyl-2-octen-1-ol was obtained in a similar way in 96% yield [172].
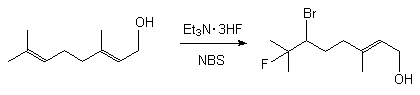
2. Processes of replacement of functional groups with fluorine.
2.1. Replacement of oxy-groups with fluorine under the influence of hydrogen fluoride complexes with bases.
Replacement of OH groups with fluorine in alcohols under the influence of anhydrous hydrogen fluoride is not a very smooth reaction due to formation of a variety of side products such as ethers, alkenes, polymers. Besides, aqueous hydrogen fluoride is a highly corrosive substance and only a special apparatus may be used in this case. Under common conditions primary alcohols do not react with anhydrous hydrogen fluoride, but at elevated temperatures or in the presence of catalysts they can be converted to fluoroalkyls [220-224]. For example, methyl fluoride can be obtained in a reaction of methyl alcohol with hydrogen fluoride in gas phase in the presence of aluminium trifluoride (at a temperature of 375-425oC [225]) or of chromium trifluoride ( 260oC). At the same time interaction of primary alcohols ( hexanol-1, octanol-1, 2,2-dimethylpropanol-1) with HF/Py complexes or with Et3N/3HF in the presence of fluorides of alkali metals results in formation of appropriate 1-fluoroalkanes in 30-55% yield [226].
The complexes of HF with bases are used as fluorinating agents for replacement of alcohol oxy-group for alcohols of relatively low reactivity, i.e. for secondary and tertiary alcohols or for benzyl alcohol [2, 227]. The review on using Py* 9HF system ( the Ohle reagent) for replacement of a hydroxy-group in secondary and tertiary alcohols with fluorine has been given in [228].
Secondary and tertiary alcohols interact with anhydrous hydrogen fluoride already at low temperatures (0oC) to form fluorides of alkyles. But there were observed products related to polymerisation. If the reaction is carried out in the presence of formaldehyde then ethers are formed. So, interaction of (CF3)2CHOH with CH2O in a medium of HF at 60-60oC results in formation of fluoromethylhexafluoroisopropyl ether FCH2OCH(CF3)2 in 90% yield which is used as anaesthetic [229]. The use of HF/Py complex in a reaction with secondary and tertiary alcohols gives better results (table 16) [2,38,230].
Hydrofluorination of amino-alcohols of PhCH(OH)CHRNR1R2 type ( where R=H,D,Me; NR1R2= piperidino; R-Me, NR1R2=morpholino; R=R1=R2=Me, R=Ph, NR1R2=piperidino) under effect of HF/Py system results in formation of a mixture of threo- and erythro-fluoroamines PhCHFCHRNR1R2 , in which the threo-isomer prevails independently on the configuration of the initial aminoalcohol [231]. In hydrofluorination by HF/Py system there is possible rotation around the C-C bond in the carbcation generating followed by hydrogen fluoride attack from the amino-group side to form intermediate carbcations G and D being in dynamic equilibrium.
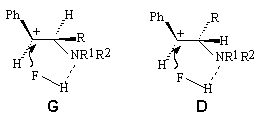
Preferable formation of the threo-product is caused by the equilibrium shift to the side of the more stable intermediate carbcation D in which steric interactions of substituents are minimal.
The hydroxyl group in the benzyl position is replaced with fluorine by 50-80% under the influence of 70% HF/Py system [232]. Thus, 1-chloro-2-hydroxy-2-phenylethane reacts with HF/Py at room temperature to give 1-chloro-1-fluoro-2-phenylethane.
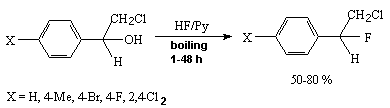
In case of  -isopropylbenzyl alcohol
in dependence on the mole ratio of HF and pyridine in HF/Py system the formation of
-isopropylbenzyl alcohol
in dependence on the mole ratio of HF and pyridine in HF/Py system the formation of 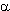 -
and
-
and 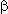 -fluorides takes place
[233].
-fluorides takes place
[233].
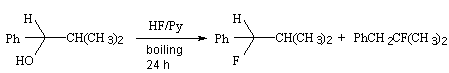
| XHF | ||
| 0,82 | 100% | 0% |
| 0,85 | 50 | 50 |
| 0,87 | 0 | 100 |
The interaction of benzyl 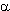 ,
,
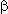 -aminoalcohol and HF/Py system results in the formation of
-aminoalcohol and HF/Py system results in the formation of 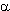 ,
,
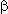 -fluoroamines [234].
-fluoroamines [234].
Table 16. Production of alkyl fluorides from secondary and tertiary alcohols under the influence of HF/Pr [2].
|
R |
ToC | Time,h | Yield,% |
| Pri | 50 | 3 | 30 |
| Bus | 20 | 3 | 70 |
| But | 0 | 1 | 50 |
| CEt3Me | 0 | 0,5 | 95 |
| CEtMeBu | 0 | 2 | 35 |
| CPr2Me | -70 | 0,5 | 85 |
| Cy | 20 | 2 | 99 |
| Norborn-2-yl | 20 | 1 | 95 |
| Adamant-2-yl | 20 | 1 | 95 |
| Adamant-2-yl | 20 | 1 | 98 |
| CHPhMe | 20 | 0,5 | 65 |
| CPh3 | 20 | 1 | 76 |
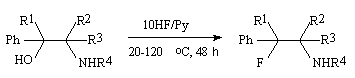
| R1,R2,R3 = Me, R4 = H |
82% |
| R1 = Et, R2,R3 = Me, R4 = H |
100 |
| R1,R2 = H, R3,R4 = Me |
95 |
| R1,R2 = Et, R3,R4 = -(CH2)- |
82 |
| R1 = H, R2 = Et, R3,R4 = -(CH2)- |
65 |
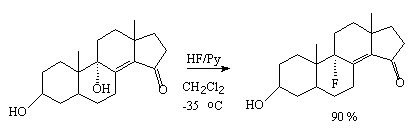
The steroids containing the OH-group , 14 and 15 groups under the
effect of HF/Py complex convert to tertiary fluoro- derivatives 16 only (9
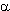 -fluoro-derivatives) [235].
-fluoro-derivatives) [235].
When secondary and tertiary OH groups are present the tertiary group is replaced with a fluorine atom
[236-238]. Thus,
3 ,9
,9
 -dihydroxy-5-
-dihydroxy-5-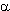 -cholest-8(14)-en-15-one
under the effect of HF/Py complex in methylene chloride gives 9
-cholest-8(14)-en-15-one
under the effect of HF/Py complex in methylene chloride gives 9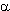 -fluoro-3
-fluoro-3
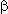 - hydroxy-5-
- hydroxy-5-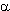 -cholest-8(14)-en-15-one
[236].
-cholest-8(14)-en-15-one
[236].
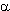 -D-Glucopyranoses containing OH
or OR groups substitute them with fluorine in position1 under the effect of HF/Py complex already
at room temperature for 10 hours [239,240]. An addition of acetone, methene chloride or collidine
brings to an increase of total yield of the reaction product.
-D-Glucopyranoses containing OH
or OR groups substitute them with fluorine in position1 under the effect of HF/Py complex already
at room temperature for 10 hours [239,240]. An addition of acetone, methene chloride or collidine
brings to an increase of total yield of the reaction product.
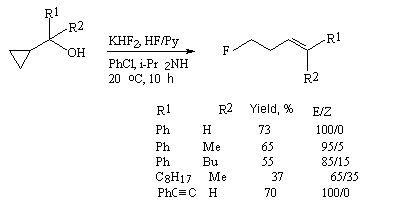
In case of alcohols containing a cyclopropane ring in a-position the influence of HF/Py/KHF2/Et2NH system brings not only to replacement of the hydroxyl group with fluorine but to opening the cyclopropane ring also [241].
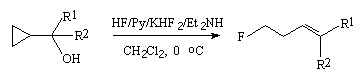
| R1 | R2 | Yield, % | E/Z ratio |
| Ph | Me | 65 | 95/5 |
| Ph | Bu | 55 | 85/15 |
| C8H17 | Me | 37 | 65/35 |
| PhCH=CH | H | 70 | 100/0 |
Table 17 shows the examples of alcohols which have substituents at the cyclopropane ring.
Interaction of alcohols containing the cyclopropane ring in 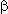 -position
with HF/Py complex or with KHF2/isopropilamine system results in formation of homo-allyl
fluorides with prevalence of the product with E-conformation [242-244].
-position
with HF/Py complex or with KHF2/isopropilamine system results in formation of homo-allyl
fluorides with prevalence of the product with E-conformation [242-244].
To be continued
Fluorine Notes, 2002, 21, 1-2
