Fluorine Notes, 2002, 20, 7-8
NEW METHOD OF SYNTHESIS OF TRIFLUOROSTYRENE
S. Igumnov, K. Narinian, E. Igumnova, A. Gontar
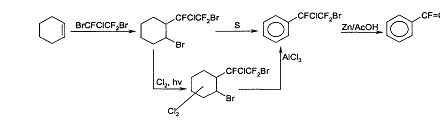
Earlier we suggested 2 ways of synthesis of Trifluorostyrene based on the interaction of cyclohexene with dibromotrifluorochloroethane with the further aromatisation of the cyclohexane ring [1]
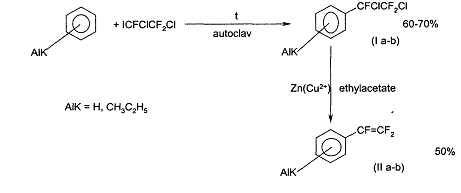
As a continuation of the research works for the development of the simple method of synthesis of trifluorostyrene and other aromatic compounds with the trifluoroethylene group, reactions of 1-iodo-1,2-dichlorotrifluoroethane with benzene, toluene and ethylbenzene were studied.
It is fixed that these compounds are alkylated by iododichlorotrifluoroethane (150-180°C, autoclave) with formation of the corresponding trifluorodichloroethyl benzenes. ( la-b)
Trifluorostyrenes ( II a-b) are formed under treatment of Zn/Cu couple of the compound (I a-b) in different solvents (acetic acid, ethanol, acetone, ethylacetate).
1. S. Igumnov, E. Igumnova, A. Gontar. 16th International Symposium, Fluorine Chemistry, Durham, UK. 2000. Pages 2p-8.
Fluorine Notes, 2002, 20, 7-8
