Fluorine Notes, 2002, 20, 1-2
Use of hydrogen fluoride and its complexes with bases for introduction of fluorine atoms into organic molecules
G.G.Furin
Novosibirsk Institute of organic chemistry named after N.N.Vorozhtsov Siberian branch of the Academy of Science of Russian Federataion
Fax: +7-3822-344752
e-mail: furin@nioch.nsc.ru
Annotation
The review summarizes and systematizes up-to-date data on fluorinating ability of anhydrous hydrogen fluoride and its complexes with bases of unsaturated organic compounds, alcohols, diazoketones, hydrazones and oximes of ketones, 3,3-dialkyl-1-aryltriazenes, aryldiazosulfides etc.. It contains an analysis of main achievements in use of anhydrous hydrogen fluoride as a fluorinating agent to produce ozone-friendly freons in gas and liquid phases both without catalysts and in the presence of latter. There has been examined factors influencing opening three-membered cycles containing oxygen and nitrogen atoms. The review contains examples of practical application of different groups of fluoroorganic compounds, rational methods of their production and their role in development of modern industry .
Contents
Introduction. Hydrogen fluoride as a basic stock substance in chemical industry.
1. Fluorination with anhydrous hydrogen fluoride and its complexes with bases of compounds from different classes.
1.1.Hydrofluorination of unsaturated compounds
1.1.1. Influence of anhydrous hydrogen fluoride on unsaturated compounds
1.1.2. Hydrofluorination of unsaturated compounds by hydrogen fluoride complexes with
bases
1.1.3. Reactions of anhydrous hydrogen fluoride and its complexes containing bases with
acetylene derivatives
1.1.4. Fluorination of alkenes with hydrogen fluoride in the presence of catalysts
1.1.5. Fluorination
of unsaturated compounds with hydrogen fluoride in the presence of electrophilic reagents
2. Processes of replacement of functional groups with fluorine atoms.
2.1. Replacement of oxy-group with fluorine under effect of hydrogen fluoride complexes containing bases.
2.2. Reactions with hydroxylamines, hydrazones and oximes of ketones.
2.3. Reactions with diazoketones,
3,3-dialkyl-1-aryltriazenes and aryldiazosulfides.
2.4. Exchange reactions of haloids under effect
of hydrogen fluoride in the presence of catalysts
3. Opening nitrogen- and oxygen-containing three-membered heterocycles
3.1.Opening an epoxy ring by anhydrous hydrogen fluoride and its complexes with bases.
3.2. Opening
nitrogen-containing three-membered heterocycles.
Conclusion.
1. Fluorination with anhydrous hydrogen fluoride and its complexes with bases of different class compounds
Hydrofluorination with anhydrous hydrogen fluoride can be carried
- in gas phase over catalysts
- in liquid phase in the presence of antimony halides
- as a liquid-phase non-catalytic process
- hydrofluorination with hydrogen fluoride/base complexes is used to be carried in liquid phase using polar solvents.
- hydrofluorination of organic compounds under the influence of anhydrous hydrogen fluoride or its complexes with bases,
- interaction of C-carbcation , generated primarily by the effect of electrophilic agent on unsaturated substrate, with solvated fluoride-ion
- direct effect of fluoride-ion upon the initial substrate.
Hydrofluorination in gas and liquid phase in the presence of catalysts is widely used in production of various chladones. The experimental date file on these processes is very huge and this review considers only new data on synthesis of ozone safety chladones without chlorine and bromine atoms. In these cases halogenated olefins and alkanes are used as initial substrates.
Anhydrous hydrogen fluoride is a relative strong acid which is inert towards oxidizers and reducing agents while it is a good dissolvent for many organic compounds. A low boiling point of hydrogen fluoride allows to isolate it after reaction and then to introduce it again into the process. These properties of hydrogen fluoride are widely used in organic synthesis for reaction medium combining properties of acid reagent and solvent.
Stability towards reducing processes makes hydrogen fluoride a perfect medium for processes of hydrogenation [19,20]. Besides anhydrous hydrogen fluoride possesses a catalytic effect in hydrogenation of different aromatic compounds, aliphatic ketones, esters of carbonic acids and anhydrides in the presence of platinum dioxide [20].
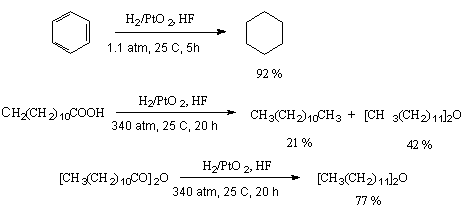
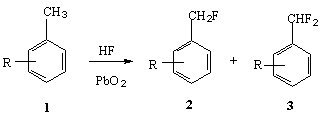
A classical example of hydrogenation of nitro-aromatic compounds in a medium of anhydrous hydrogen fluoride runs under mild conditions to form appropriate anilines. It should be noted that sometimes introduction of fluorine into benzene ring takes place and the reasons of that will be discussed below [21,22].
Fluorination of the C-H bonds in the hydrocarbon chain under influence of hydrogen fluoride takes place only in the presence of oxidizers and is realized only for benzene derivatives. A number of such processes is realized in a medium of hydrogen fluoride. Thus, there has been realized oxidizing fluorination of the C-H bond for toluene [23] and ethyl benzene derivatives [24] by hydrogen fluoride in the presence of lead oxide [23], NiO2 [23], AgF2 [24], CoF3 [24], Co(OAc)3 [24].
| R | PbO2 Mmol |
T, oC | Time, hrs | Yield, % | |
| 2 | 3 | ||||
| 4-NO2 | 10 | 25 | 48 | 4 | 11 |
| 2-NO2 | 30 | 25 | 5 | - | 40 |
| 4-CN | 30 | 25 | 1 | - | 46 |
| 4-COOH | 30 | 25 | 3 | - | 60 |
| 4-CONH2 | 30 | 0 | 1 | 88 | 9 |
| 3,4(NO2)2 | 30 | 25 | 1 | 75 | - |
But HF-PbO2 system in case of absence of alkyl groups can fluorinate the benzene ring to give a cyclohexadiene structure [25]. So, interaction of phenol with 70% HF/Py or KF/HF system in the presence of PbO2 results in formation of 4,4-difluorocyclohexa-2,5-dienone [25].
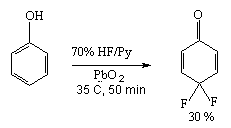
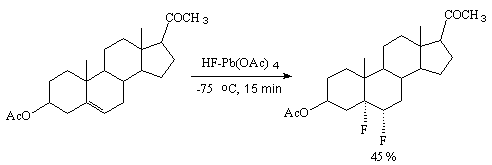
In case of unsaturated organic compounds the reaction with anhydrous hydrogen fluoride in the presence of PbO2 or Pb(OAc)4 results in the fluorine adduct to the double bond without replacement hydrogen or chlorine atoms [26].

Steroids are fluorinated by this system regioselectively to form cis-difluorosteroids [27].
Anhydrous hydrogen fluoride is an excellent reagent for intermolecular cyclizations. So, diethylarylmalonate gives tetralone derivatives in good yield [28].
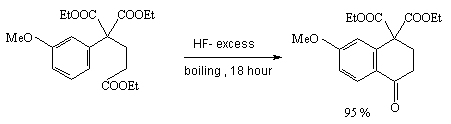
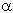 -Benzamidoacetophenone
turns into 2,5 diphenyloxazoline [29]
-Benzamidoacetophenone
turns into 2,5 diphenyloxazoline [29]
Hydrogen fluoride in acetonitrile is a good catalyst for intermolecular Diels-Alder reactions
[30]. This reagent promotes high stereoselectivity of cyclization of triene ethers while the
use of different acids, HCl, HAc, CF3COOH results in polymerization products.
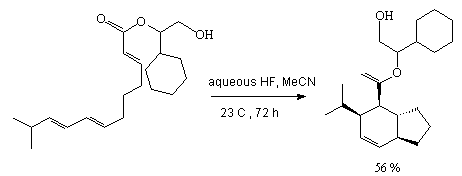
It is important that hydrogen fluoride is convenient to use in industrial technologies because
of its relative cheapness and easiness in handling in gas-phase flow continuous systems over
catalysts and in liquid-phase processes. But these reactions require apparatus designed for high
pressure and high temperature.
It is relatively easy to replace haloids atoms bound with a double bond or benzene ring. Thus, conversion of benzotrichloride to benzotrifluoride, the main semi-product for synthesis of pesticides and medicine preparations, under influence of anhydrous hydrogen fluoride runs within a comparatively wide temperature interval in high yields [31-33].
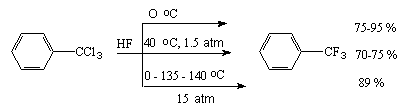
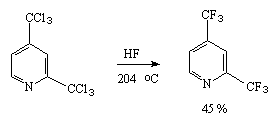
An analogous reaction has been implemented for chlorinated homologues of pyridine also [34].
1.1. Hydrofluorination of unsaturated compounds.
Fluorinating properties of anhydrous hydrogen fluoride are determined by the presence of effective
fluoride-ion [ HnF n+1]-. Reactions of fluorination of organic
compounds under influence of hydrogen fluoride or systems containing hydrogen fluoride include
generation of cationoid particles at protonation of the 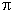 -bond
or heteroatom containing unshared electron pair. Further this intermediate is subjected to the
attack of solvated [ HnF n+1]- fluoride ion. The nature of the
used solvents plays an important role. A low solubility of hydrogen fluoride in a number of solvents
(Et2O,CCl4, alkanes) results in formation of a two-phase system and effective
interphase catalysis promotes the reaction course [35-48].
-bond
or heteroatom containing unshared electron pair. Further this intermediate is subjected to the
attack of solvated [ HnF n+1]- fluoride ion. The nature of the
used solvents plays an important role. A low solubility of hydrogen fluoride in a number of solvents
(Et2O,CCl4, alkanes) results in formation of a two-phase system and effective
interphase catalysis promotes the reaction course [35-48].
The use of hydrogen fluoride as
the most prevalent fluorinating reagent can be subdivide into the following groups:
1. hydrogen fluoride as a catalyst and fluorinating agent,
2. hydrogen fluoride as a medium and reagent of fluorination in the presence of catalysts for implementation of exchange processes. This approach is used for production of chladons,
3. hydrogen fluoride as a medium for carrying out processes of fluorination under influence of elemental fluorine and other strong fluorinating reagents
4. hydrogen fluoride as a medium and reagent for carrying out electrochemical fluorination. This method is assumed as a basis for production of perfluorinated organic compounds.
1.1.1. Influence of anhydrous hydrogen fluoride on unsaturated compounds.
The influence of hydrogen fluoride upon alkenes is one of the most important reactions of synthesis of secondary and tertiary alkylfluorides. Usually under the effect of anhydrous hydrogen fluoride an electrophilic addition according to Markovnikov rule with formation of a monofluoro-derivative takes place. Due to the ability of HF to speed up polymerization of olefins [49,50] this reaction in many cases is followed by side formation of polymers. As a rule the addition of HF to an olefin results in a great amount of a polymer while the addition of an alkene to HF excess results in formation of fluorine-containing compounds in high yield.
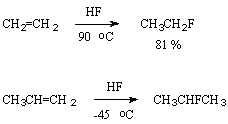
The addition of hydrogen fluoride to olefins is a classical example of introduction of one fluorine atom. Hydrofluorination of olefins under the influence of hydrogen fluoride is determined by the nature of substituents at the multiple bond and often depends on the use of a catalyst or solvent promoting ionization. So, fluoroolefins possessing electrophilic properties react at first with fluoride ion to give an intermediate carbanion , while olefins with pronounced nucleophilic properties due to electron-donating substituents are protonated on the contrary to give stable intermediate carbcations because HF is a rather strong acid for that.
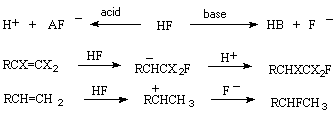
A big data file can be united into the following groups:
Fluoroethane is obtained in gas-phase hydrofluorination of ethylene under pressure under the effect
of HF [35,50]. Propylene is hydrofluorinated in liquid phase under influence of HF (0oC,
2 hours, 35%, 60% yield) [50] or of HF/Py (20oC, 1 hour, 35% yield) [50,51]. Butene
gives 2-fluorobutane [46,47], 2-methylpropene results in 2-fluoro-2-methylpropane [52]. It is
assumed that the both reactions runs through formation of a complex of one or two molecules of
hydrogen fluoride with the  -bond
[53].
-bond
[53].
Interactions of anhydrous hydrogen fluoride with buten-2,2-methylpropene, 2-methylbutene-2 give 2-fluorobutane, 2-fluoro-2-methylpropane and 2-fluoro-2-methylbutane in high yield [54]. In case of propene 2-fluoropropane is obtained in 60-75% yield at 0-45oC
Cyclohexene reacts with hydrogen fluoride already at –78oC to give fluorocyclohexane in 70% yield while a temperature increase ( above 100oC) brings to cyclohexene polymerization [49,50].
Interaction of allene and hydrogen fluoride at –70oC results in formation of 2,2-difluoropropane in 50% yield [55,56].
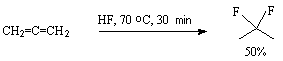
Comparison of behavior of ethylene and cyclohexene shows that in the first case the fluoroethane yield is increasing with temperature increase while the fluorocyclohexane yield drops on the contrary (table 2).
Anhydrous hydrogen fluoride contains fluoride ion not active enough to realize hydrofluorination of perfluoroolefins though such examples exist. For example, hydrofluorination of hexafluoropropylene [57].
Table 2. Fluorination of olefins with hydrogen fluoride [49].
|
T,oC |
Yield ,% |
||
| 2-Fluoroethane | 2-Fluoropropane | Fluorocyclohexane | |
| -60 | 0 | ||
| -45 | 62 | ||
| -35 | 80 | ||
| 0 | 22-27 | 42-61 | |
| 10 | 54 | ||
| 25 | 45-55 | ||
| 35 | 10 | ||
| 75 | 2 | ||
| 90 | 81 |
Hydrofluorination of hexafluoropropylene with hydrogen fluoride runs more effectively under
pressure (0.33-0.35 kg/cm2) and a ratio of HF: hexafluoropropylene= 1.3 -1.5 at a
temperature above 210oC [58]. So 2H-heptafluoropropylene (freon 227) is obtained in
98.5% yield. Isolation of the desired product becomes easier because fluoroolefins with HF give
azeotrops which are easy to distillate from the reaction product [56,59].
1,1,1,2-Tetrafluoro-2-propene under the effect of HF gives 1,1,1,3,3-pentafluoropropane in a good yield [60]. At the same time when a chlorine atom presents at the multiple bond , its replacement with a fluorine atom takes place. Thus hydrofluorination of 1,1,1-trifluoro-3-chloro-2-propene with anhydrous hydrogen fluoride results in formation of 1,1,1,3-tetrafluoro-2-propene [60].
The latter under the reaction conditions then turns out into 1,1,1,3,3-pentafluoropropane in the excess of HF [61].
The hydrofluorination process in series of ethylene derivatives runs similarly. For example under the effect of anhydrous hydrogen fluoride upon trichloroethylene a total exchange of chlorine atoms with fluorine takes place to form 1,1,1,2-tetrafluoroethane in high yield and selectivity [62]. Probably the reaction runs through intermediate formation of 1-chloro-2,2,2-trifluoroethane. This compound under the influence of HF gives 1,1,1,2-tetrafluoroethane as a main product [62].
Hydrofluorination of perchloroethylene with anhydrous hydrogen fluoride gives a mixture of products with pentafluoroethane as a main compound [63].
Non-catalytic process of hydrofluorination is widely used for production of chladons (freons). For example, synthesis of 143a, 142b, 141b chladons contains fluorination of vinylidene chloride or methylchloroform with hydrogen fluoride under conditions of recirculation of unreacted products [64].
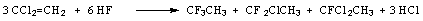
Hydrofluorination of 1,1-dichloroethylene in liquid hydrogen fluoride proceeds in 3 stages, at first at 63-83oC and under a pressure of 4.0-7.0atm, then at 120-130oC and 16.6-20.2 atm pressure. The hydrofluorination ends at 130oC and under a pressure of 20.2 atm to give 1-fluoro-1,1-dichloroethane and 1,1-difluoro-1-chloroethane [65,66]. The behavior of trichloroethylene is similar.
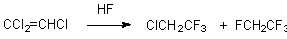
1,1,1,2-Tetrafluoroethane (134a freon) is obtained by a reaction of trichloroethylene with anhydrous hydrogen fluoride at 250oC and a pressure of 4 atm ( a ratio of reagents of 6:1) in 85.4% yield [67]. 2-H-Heptafluoropropane is formed in high yield under the influence of anhydrous hydrogen fluoride on hexafluoropropylene in the presence of weak alkaline ion-exchange resins containing a tert-amino group [57], though it is possible to carry out the process without catalyst but at a higher temperature [56].
It is rather effectively to initiate by laser the hydrofluorination of unsaturated compounds (acetylene, 1,3-butadiene, 1,1-difluoroethylene, trifluorochloroethylene) with anhydrous hydrogen fluoride [68].
Tri-O-benzoyl-D-glucal in hydrofluorination with anhydrous hydrogen fluoride at –60oC
gives 3,6-di-O-benzoyl-2-desoxy- -D-ribohexapyranozyl
fluoride [69].
-D-ribohexapyranozyl
fluoride [69].
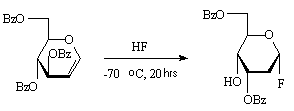
Synthesis of fluorosteroids can be realized by addition of hydrogen fluoride to the double bond of
pregnene and cholestene derivatives. Here stereochemistry and the substituent nature play an
important role [70-77]. For example, the formation of 5-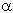 -fluoroderivative
:
-fluoroderivative
:
1.1.2. Hydrofluorination of unsaturated compounds with complexes of hydrogen fluoride with bases. To overcome difficulties in hydrofluorination of alkenes with anhydrous hydrogen fluoride it was proposed to use solutions of amines in hydrogen fluoride. That led to stable liquid complexes and unification of hydrogen fluoride properties [2]. On the example of cyclohexene there was studied the influence of amine series with HF such as follows: Et3N, BuNH2, Bu2NH, C6H13NH2, pyperidine, PhNH2, PhNMe2, 1,2-(NH2)2C6H4, pyridine and melamine [3]. The yields of fluorocyclohexane at 0oC, 1 hour were equal to: 12, 37, 33, 28, 9, 50, 62. 11, 80 and 90% accordingly.
A higher yield and selectivity is reached in hydrofluorination using complexes of hydrogen fluoride with bases (for example, 70% HF/Py [2]. 60% hydrogen fluoride-polyvinylpyridine [42], 86% hydrogen fluoride/melamine [36,46], Table3).
The yield of fluoroalkanes is increased at transition from HF/Py system to HF/melamine system (14% of melamine and 86% of hydrogen fluoride) [40,46]. A complex of triethylamine/hydrogen fluoride (Et3N/3HF) possesses high selectivity in hydrofluorination and is used in producing tertiary alkylfluorides [78,79]. Besides, for hydrofluorination of alkenes there was used a complex of imidofluoride-HF [R(CF=NH2)+ F- nHF (R= Ph, alkyl, haloalkyl)].
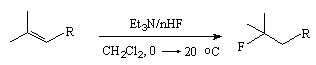
|
R |
n | Time, hours | Yield,% |
| (CH2)8CH=CH2 | 5 | 72 | 70 |
| (CH2)3CH=CHEt | 5 | 24 | 78 |
| (CH2)2Ac | 5 | 30 | 82 |
| (CH2)2CHMe(CH2)2 | 6 | 48 | 76 |
| (CH2)2MeC=CHCOOEt | 5 | 30 | 81 |
Table 3. Hydrofluorination of alkenes with anhydrous hydrogen fluoride [49,51]
| Substrate | method | T,oC | Time, hours | Product | Yield,% |
| Propene | A | 20 | - | 2-fluoropropane | 35 |
| Cyclopropene | A | 20 | - | 1-fluorocyclopropane | 75 |
| Butene-2 | A | 0 | - | 2-fluorobutane | 40 |
| 2-methylpropene | A | 0 | - | 2-fluoro-2-methylpropane | 60 |
| 2,3-dimethylbutene | B | 15 | 6 | 2-fluoro-2,3-dimethylbutane | 72 |
| Cyclopentene | A | 0 | - | fluorocyclopentane | 65 |
| Cyclohexene | A | 0 | - | fluorocyclohexane | 80 |
| B | 20 | 2 | 76 | ||
| C | 0 | 0.1 | 88 | ||
| 1-methylcyclo-hexene | B | 0 | 1 | 1-fluoro-1-methylcyclohexane | 80 |
| Norbornene | A | 0 | - | 2-fluorobornane | 65 |
| B | 0 | 1 | 79 | ||
| Cycloheptene | A | 0 | - | fluorocycloheptane | 90 |
| B | 0 | 1 | 81 |
A - HF/Py
B - HF/polyvinylpyridine, methylene chloride
C - HF/melamine [40]
In hydrofluorination of dienes with a system of Et3N/5HF or 6HF a double bond having volume substituents is affected selectively [78,79]:

A solution of melamine in anhydrous HF is widely used as a highly effective and convenient hydrofluorinating agent of alkenes and alcohols [47]. It can be used in a mixture with solvents (pentane or CCl4). The yield of the desired product is 99% at 100% selectivity. The presence of small amount of moisture does not influence negatively.
HF complexes with a number of nitrogen-containing heterocycles were found effective hydrofluorinating agents. Besides under their influence there is possible exchange of chlorine with fluorine in the CCl3 group. For example, a mixture of 1,3-dimethyl-2-imidazolydinone hydrofluoride and 4-chloro-3,5-bis(trichloromethyl)pyrazole at heating on an oil bath gives 4-chloro-3,5-bis(trifluoromethyl)pyrazole in good yield [80].
The reaction of alkenes with hexamethyltetra amine in anhydrous hydrogen fluoride gives 1,3,5-
trisubstituted hexahydro-1,3,5-triazine which hydrolysis under mild conditions results in
formation of  -fluoroalkylamines
[26,81].
-fluoroalkylamines
[26,81].
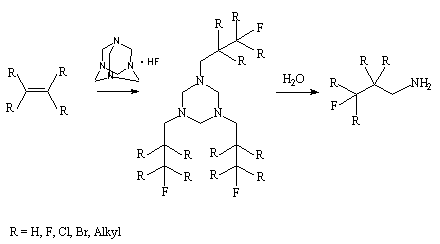
To increase its activity there is used a complex of hydrogen fluoride with N,N, N', N'-tetramethylnaphtaline-1,8-diamine (A-HF) [20]. The carbanions generating in the process are used for oligomerization of perfluoroalkenes and for reactions of perfluoroalkylation of polyfluoroaromatic [82] and heterocyclic [83] compounds with perfluoroolefins.
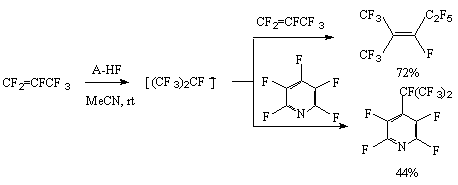
There was found a possibility to carry out hydrofluorination with solid acid potassium fluoride in the presence of silicon tetrafluoride at room temperature [84].
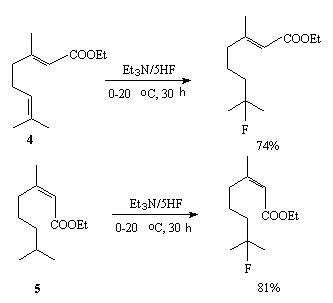
Natural compounds such as compounds 4 and 5 containing multiple bonds are fluorinated selectively with a complex Et3N/5HF under mild conditions [78]. In this the multiple bond at which are electron-donor substituents is affected.
In bicyclic terpenes under influence of hydrogen fluoride there is observed a rearrangement affecting
the multiple bond. So hydrofluorination of camphene under the effect of hydrogen fluoride
at –80oC gives a mixture of iso-bornil fluoride and camphene hydrofluoride.
Bromofluorination of fluorovinyl analog of 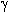 -aminobutanic
acid is of interest from biological point of view.
-aminobutanic
acid is of interest from biological point of view.
1.1.3.Reactions of anhydrous hydrogen fluoride and its complexes with bases with acetylene derivatives.
A similar picture takes place in acetylene hydrofluorination also. Acetylene reacts with anhydrous hydrogen fluoride in liquid phase without catalyst to give vinyl fluoride and 1,1-difluoroethylene. It is better to carry this process in the presence of catalysts (table 4)
Table 4. Hydrofluorination of acetylene with anhydrous hydrogen fluoride over catalysts.
|
Temperature,oC |
Catalyst | Yield, % |
References |
|
| CH2=CHF | CH3CHF2 | |||
| 70a | PhN(CH3)2, Ph2Hg | 97 | 87 | |
| 55a | KBF4, HSO3F | 99,7 | 88 | |
| 20a | HSO3F, K2ZrF6 | 99,6 | 89 | |
| 50b | Hg(NO3)2, Cd(NO3)2 on carbon | 98 | 1 | 90 |
| 300b | Cd(BF4)2 on Al2O3 | 91 | 91 | |
| 100b | HgCl2, BaCl2 on carbon | 82 | 4 | 92 |
| 350b | AlF3 | 80 | 13 | 93 |
| 310b | SbF3 on Al2O3 | 26 | 70 | 94 |
| 275b | AlF3 | 19 | 81 | 95 |
| 230b | Al2O3 , silicic acid | 2 | 98 | 96 |
| 200 | Al2O3 | >90 | 97 | |
| 262b | H3BO3, Fe2O3 | 99 | 98 |
It should be noted that in catalytic hydrofluorination of acetylene it is managed to get fluoroethylene, a primary adduct of one HF equivalent to the triple bond, which is then hydrofluorinated to form 1,1-difluoroethane [99-108].
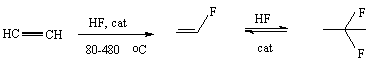
As catalysts there were used ( in liquid phase) HSO3F/SbF5 [104], HSO3F or RfSO3H/K2TiF6, KAsF6, Na3AlF6, Na2SiF6, K2SnF6 [106].
Phenylacetylene enters into reactions with hydrogen fluoride with more difficulties in comparison with acetylene. Mercury oxide on carbon was used as a catalyst. The yield of 1,1-difluoro-1-phenylethane was only 18%.
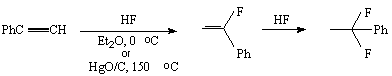
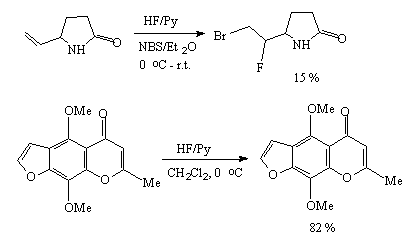
The complexes of hydrogen fluoride with bases are effective fluorinating agents for conversion of inactivated alkynes to hemi difluoro-derivatives [1]. The reaction of hydrogen fluoride addition to acetylene was widely studied due to formation of vinyl fluoride, an important commercial monomer. Acetylene reacts with hydrogen fluoride in liquid phase without catalyst to form vinyl fluoride and 1,1-difluoroethane. But more acceptable results were obtained in the presence of catalysts. So hex-1-enyl and hex-3-enyl with hydrogen fluoride-polyvinylpyridine give 2,2-difluoro- ( in 56%) and 3,3-difluorohexanes ( in 59% yield). But the reaction time is long enough, up to 72 hours [2].
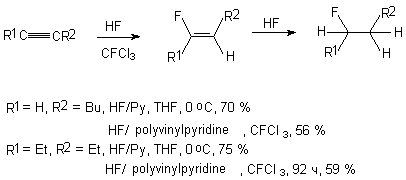
Alkylacetylenes form hemedifluorides in a reaction of hydrogen fluoride in a medium of diethylether
at –50 - 0oC or in a reaction with HF/Py system in tetrahydrofuran [2].
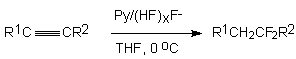
R1 = H, R = 2 = Bu 70 %
R1 = R2 = Et 75 %
Electron-negative substituents of acetylene, for example, dimethyl acetylene-dicarboxylate do not react with hydrogen fluoride under usual conditions while in the presence of fluoride-ion sources (CsF, tetra-alkyl ammonium dihydrofluoride) under conditions of inter-phase catalysis they give products of addition of one molecule of hydrogen fluoride to the triple bond [76].
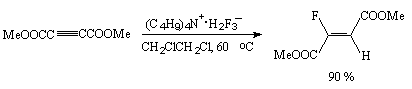
Direct addition of hydrogen fluoride to the triple bond runs according to the Markovnikov law and results in formation of difluoro-derivatives.Some examples are given in table 5. The use of tetrabutylammonium dihydrotrifluoride as a hydrofluorinating agent brings to formation of products of addition of one equivalent of HF to the triple bond that has made a ground for a method to obtain derivatives of olefins containing one fluorine atom at the double bond. Dihydrotrifluorides of tetrabutylammonium (Bu4N+*H2F3-) or of dihydrofluoride (P+*H2F3-)[P+*F-, P+ is a polymeric cation made of microporous anion-exchange resin] on a polymer carrier (for example, amberlite JRA900) have been found very convenient hydrofluorinating agents and are used for electrophilic alkynes[110,114,115].In this case only one molecule of hydrogen fluoride is added to the triple bond.
HF/Py complex was found convenient in hydrofluorination of acetylene derivatives [13,51].
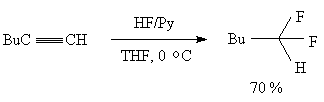
An interesting way of fluorination was proposed by the authors [113,116] who fluorinated acetylene derivatives by a system of Py/10HF in the presence of nitrosyl tetrafluoroborate.
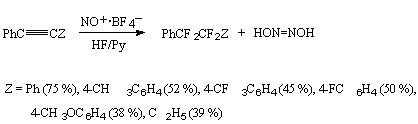
It is assumed that the reaction runs through a primary attack towards the triple bond of nitrosyl
cation carbon atom according to the scheme:
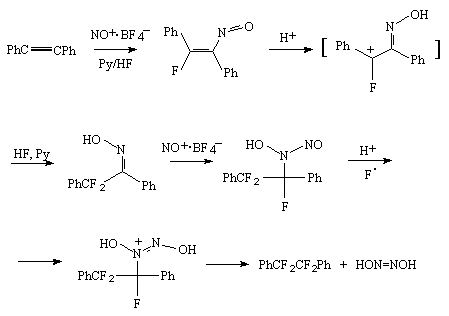
Table 5. Hydrofluorination of acetylene derivatives with anhydrous hydrogen fluoride.
| Alkyne | Product | Yield,% | References |
MeC CH CH |
MeCF2Me | 61 | 99 |
EtC CH CH |
EtCF2Me | 75 | 99,100 |
MeC CMe CMe |
EtCF2Me | 75 | 99,100 |
PrC CH CH |
PrCF2Me | 75 | 99,100 |
BuC CH CH |
BuCF2Me | 75 | 99,100 |
EtC CEt CEt |
PrCF2Et | 75 | 99,100 |
Me(CH2)4C CH CH |
Me(CH2)4CF2Me | 75 | 99,100 |
Me(CH2)5C CH CH |
Me(CH2)5CF2Me | 75 | 100 |
PrC CPr CPr |
BuCF2Pr | 75 | 100 |
Table 6. Hydrofluorination of acetylene substituents Bu4NF 2HF [110-113]
Alkyne Conditions Product Z/E ratio Yield,% T,oC Time,h MeOOCC
 CCOOMe 60 9 MeOOCCF=CHCOOMe 100:0 90 PhC
CCOOMe 60 9 MeOOCCF=CHCOOMe 100:0 90 PhC Bz
110 50 PhCF=CHBz 100:0 53 Me(CH2)6C
Bz
110 50 PhCF=CHBz 100:0 53 Me(CH2)6C CCN
110 7 Me(CH2)6CF=CHCN 70:30 95 BuC
CCN
110 7 Me(CH2)6CF=CHCN 70:30 95 BuC CCOOMe
120 24 BuCF=CHCOOMe 42:58 90 PhC
CCOOMe
120 24 BuCF=CHCOOMe 42:58 90 PhC CCOOMe
120 21 PhCF=CHCOOMe 91:9 75 PhC
CCOOMe
120 21 PhCF=CHCOOMe 91:9 75 PhC CCHO
110 4,5 PhCF=CHCHO 91:9 75
CCHO
110 4,5 PhCF=CHCHO 91:9 75
1.1.4.Fluorination of alkenes with hydrogen fluoride in the presence of catalysts
Hydrofluorination in gas and liquid phases in the presence of catalysts is widely used in production of various chladones. A data file on these processes is vast and is not under consideration in this review. Some examples of such a fluorination in connection with transition to production of ozone-safety chladones not containing chlorine and bromine atoms we present in Table 7. Here chlorine-containing olefins are used as the initial substrates.
Table 7
| Olefin | Catalyst | Conditions | Product (Yield,%) | References |
| CH2=CHCl | SnCl4 | - | CH3CHF2 | 117 |
| 60-100; 6-10 atm | 118 | |||
| AlF3/Cr2O3/NiF2 | - | 119 | ||
| C/BF3 | 100 | CH3CHClF(90) | 120 | |
| VCl3/C | 225 | CH3CHF2(91) | 121 | |
| SnCl4 | 80-120; 8-15 atm | CH3CClF2 | 122 | |
| CH2=CCl2 | SnCl4/P(OEt)3 | 60 | CH3CCl2F(61) | 123 |
| CH3CClF2(34) | ||||
| O2;AlCl3;fluorides of Fe, Cr,Co | 400-700 | CH2=CF2(80) | 124 | |
| Bi(NO3)3 | 198-210 | CH3CF3(99,7) | 125 | |
| CrCl3 | 198 | CH3CF3(99) | 116 | |
| SnCl4 | Cl2FCMe(61,5) | 126,127 | ||
| Bi(NO3)3-Mn(NO3)2 | 250 | CH3CF3(100) | 128 | |
| Al,Zn,Sn,Fe fluorides | 25-75 | Cl2FCMe | 129 | |
| AlF3 | 74-86 | CH3CClF2(90) | 130 | |
| CHCl=CCl2 | WF6 | 100-120 | CH2ClCCl2F(81) | 131 |
| TaF5 | 5 | CH2ClCCl2F(89) | 132 | |
| BF3 | 95 | CH2ClCCl2F(60) | 133 | |
| TiCl3,MoCl5,WCl5,NbCl5 | 50-150 | CH2FCH2Cl (84,5) | 134 | |
| In2O3 | 350 | CH2ClCF3(91) | 135 | |
| SbClxFy | 25-60 | CH2ClCF3(93,1) | 136-138 | |
| salt of Bi,Mn | 235-250 | CH2ClCF3(92) | 139 | |
| CCl2=CCl2 | TaF5 | 150 | CHCl2CClF2(93) | 132 |
| NbF5 | 150 | CHCl2F(34) | 132 | |
| MoCl5 | 150 | CHCl2CCl2F(53) | 132 | |
| CHCl2CClF2(16) | ||||
| TiCl4 | 150 | CHCl2CCl2F(42) | 132 | |
| CHCl2CClF2(11) | ||||
| SbCl5 | 150 | CHCl2CCl2F(21) | 132 | |
| CHCl2CClF2(30) | ||||
| TaCl5 | 119-122 | CHCl2CCl2F(12) | 140 | |
| CHCl2CClF2(43) | ||||
| NbCl5 | 142-148 | CHCl2CClF2(85) | 141 | |
| CHCl2CF3(11) | ||||
| NiCl2 | 325 | CHCl2CF3(70) | 142 | |
| CHClFCF3(12) | ||||
| CH2=CHF | CrCl3 | - | CH3CHF2 | 116 |
| CHF=CF2 | Cr2O3 | 350 | CH2FCF3(97,8) | 143,144 |
| CH3CCl=CH2 | 14 | CH3CClFCH3(75) | 145 | |
| CF3CF=CF2 | CrO2F2 | 260-270 | CF3CHFCF3 | 146-148 |
| TaF5,NbF5,SbF5 | 250-350 | CF3CH2F | 144 | |
| CF2=CF2 | Cr,Mg | 310-475 | CF3CHF2 | 144 |
These processes were taken on special significance in recent years due to development of new ozone-safety freons containing atoms of C,F,H only and in connection with the Vienna convention about protection of the ozone layer (1985). Prohibition of production and consumption of chlorofluorocarbons was a ground for development of studies and technologies for production of new freons. The main attention was paid to freons which could completely replace chlorofluoro-containing freons. Table 8 presents the data on such freons.
Table 8
| Chladons (Freons) | B.p. oC | E1 | E2 | A | Toxicity, mg/m3 |
| Trifluoromethane (CHF3) | -82,2 | 0 | 3000 | ||
| Difluoromethane (CH2F3) | -51,7 | 0 | 0,13 | 13,3-29,3 | >1000 |
| 1,1,-Difluoroethane (152a) | -24,5 | 0 | 0,03 | 4,0-19,0 | 3000 |
| 1,1,1-Trifluoroethane (143a) | -47,6 | 0 | 0,76 | 7,0-19,0 | 3000 |
| 1,1,1,2-Tetrafluoroethane (134a) | -26,5 | 0 | 0,25 | no | >1000 |
| Pentafluoroethane (125) | -48,5 | 0 | 0,84 | no | >1000 |
| 1,1,1,2,3,3,3-Heptafluoropropane (227ea) | -18 | 0 | no | >1000 |
Gas-phase hydrofluorination is most effective over catalysts, the process runs under elevated temperatures and pressure. As a rule, alkenes with a different number of chlorine atoms are used as substituents. So, 1,1-dichloroethylene (50-70oC, 10-30 Bar) gives 1,1-dichloro-1-fluoroethane [149]. Trichloroethylene in a fluidized catalyst ( chromo-magnesium fluoride) in gas-phase hydrofluorination under the influence of anhydrous hydrogen fluoride gives at first 1,1,1-trifluoro-2-chloroethane (Freon 133a)[64,150]. This stage is practically irreversible. As it follows from experimental data and thermodynamic calculations of the process, the content of Freon 133a in organic part of the synthesis products can be 90-98% by volume. The optimal conditions for obtaining high yield of Freon 133a are as follows: mole ratio of HF: C2HCl3=4-7:1, a temperature of 250-350oC, a contact time of 5-15 s [64,150].
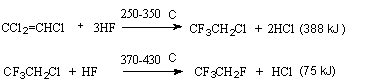
Further hydrofluorination of Freon 133a to 1,1,1,2-tetrafluoroethane (Freon 134a)
takes place [64,150]. The second stage is reversible and runs at a higher temperature
of 370-430oC ( mole ratio HF: Freon 133a = 5-15:1, a contact time is 3-10s),
the content of Freon 134a is small and equal to 20-40 %by volume.
The influence of pressure on the 1 and 2 stages of the process has been investigated [151]. It was found that in the first stage a pressure increase up to 6 atm resulted in an increase in conversion of trichloroethylene ( 95-97%), when pressure was increasing up to 3 atm the selectivity of Freon133a was increasing and further practically did not change. In the second stage a pressure increase results in a reduction of freon133a conversion : a pressure change from 1 to 3 atm brings to a change in selectivity from 22.3 to 15.8%, at higher pressures the conversion remains almost constant (14.6- 14.8%). Nevertheless industrial production of Freon 134a according to this scheme is perspective [64].
Gas phase hydrofluorination of perchloroethylene and hydrogen-containing ethanes at elevated temperatures (330-465oC) and under pressure in an excess of anhydrous hydrogen fluoride in the presence of a catalyst containing a chromium compounds and magnesium fluoride in amount of 8-24% of the catalyst mass at a mole ratio of HF:alkene= 4-40:1 and a contact time of 5-60 sec results in formation of fluorine-containing ethanes [152].
Perchloroethylene with HF in the presence of a catalyst based on Cr2O3 at 350oC and 1 bar at the contact time of 12.5 sec gives 1,1,1,2,2-pentafluoro-ethane (Freon 125) as the main reaction product [153,154].
Propylene derivatives behave similarly over a catalyst based on fluorinated aluminum: 1-chloro-3,3,3-trifluoropropylene and 1,3,3,3-tetrafluoropropylene give 1,1,1,2,2-pentafluoropropane [155]. This Freon is obtained also in hydrofluorination of 1,1,3,3-tetrachloropropylene and 1,3,3,3-tetrachloropropylene over SbF5 catalyst at a pressure of 10kg/cm2 (20 hours) [156].
Hexafluoropropylene in gas phase is hydrofluorinated under the influence of HF over catalysts based on compounds of trivalent chrome with additions of aluminum fluorides at 210oC and a ratio of reagents of 4-20:1 (HF:hexafluoropropylene) with formation of 2H-heptafluoropropane (R- 227ea) in 98.5% yield [157,158]. R-227 is a perspective ozone-safety Freon which can be used as an effective fire-extinguishing agent.
In hydrofluorination of fluoroalkenes under the influence of anhydrous hydrogen fluoride over an active catalyst, an activated carbon promoted by fluorides of alkali metals (NaF, KF, CsF, RbF), fluorocarbons are formed [159].
So, in gas phase hydrofluorination the most effective are catalysts based on compounds of aluminum [160] and chromium [161-163], magnesium, bismuth [164,165] whereas many tri- and tetrahalogen-substituted olefins add hydrogen fluoride in the presence of BF3 (60-180oC), fluorides and chlorides of tin and antimony. The reactions of halogen-containing olefins with hydrogen fluoride in the presence of catalysts are of great industrial importance and a large data file has been collected for these processes [30].
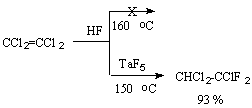
Liquid-phase hydrofluorination requires a lower temperature in comparison with the gas phase process and as catalysts there are used fluorides of transition metals in the higher oxidation degree, BF3 and super acids in HF medium. Catalytic hydrofluorination is more effective [132]. Tetrachloroethylene is not influenced by hydrogen fluoride at 160oC and reacts with them only in the presence of a catalyst, for example TaF5 [166].
Perfluorinated alkenes are also subjected to the effect of anhydrous hydrogen fluoride
in liquid phase over catalysts. Thus hydrofluorination of tetrafluoroethylene in
the presence of SbF5 and tertiary amines results in formation of pentafluoroethane
[167]. So at a content of SbF5 of 0.02 mole% and at mole ratios of tetrafluoroethylene:SbF5=15:1
and tetrafluoroethylene:SbF5 =1:10 , the contact time of 4 h, temperature
90oC there was obtained a mixture of products with the following content:
C2HF5 84.5%, C2F6 0.3%, C2F4 15.2%. Hydrofluorination of hexafluoropropylene in liquid phase in the presence of
Lewis acids ( fluorides of transition metals TaF5, NdF5, SbF5)
gives 1,1,1,2,3,3,3-heptafluoropropane [167a]. The same compound is formed when tributylammonium
hydrofluoride (Bu3N HFx (2<x<3)) is used as a catalyst [167b].
To be continued
Fluorine Notes, 2002, 20, 1-2
