Fluorine Notes, 2010, 70, 5-6
STUDYING THE ANODE BEHAVIOUR OF NICKEL IN LIQUID ANHYDROUS HYDROGEN FLUORIDE
G. I. Kaurova, V. A. Matalin, N. B. Lesnevskaya
FSUE Russian Scientific Center "Applied Chemistry", St. Petersburg, Dobrolubov av. 14
E-mail: gkaurova@rscac.spb.ru
Abstract. The anodic behaviour of nickel in liquid hydrogen fluoride was investigated. It is shown that fluorination of organic compounds was carried out by a radical of fluorine, formed as a result of the discharge of a fluorine ion, adsorbed on the passive nickel anode. Found that the rate of electrochemical fluorination is determined by diffusion limitations at the anode surface.
Keywords: Electrochemical fluorination (ECF), anode, hydrogen fluoride, charging curve.
The solutions based on liquid anhydrous hydrogen fluoride, as a rule, conduct the current well and many of them are used for laboratory practice and industry for electrochemical synthesis of fluorinated compounds [1].
Anhydrous hydrogen fluoride as a solvent has got its characteristics and in terms of some physical properties sharply differs from other haloidhydrogens, rather resembling water [2]. As water, anhydrous hydrogen fluoride possesses a high degree of association, which causes strong polarity and high dielectrical constant and their feature a capability to dissolve ionic compounds. However, as opposed to water anhydrous hydrogen fluoride is one of the most acidic solvents.
As opposed to aqueous solvent usually not a dissolved compound is dissociating during solving in anhydrous hydrogen fluoride, but a solvent [3]. Cations are complex ions formed by the proton of anhydrous hydrogen fluoride and dissolved compound (or by the product of its solvolysis). Anions of significant quantities can be only fluorine ions solvated by molecules of anhydrous hydrogen fluoride.
Due to the fact, that anhydrous hydrogen fluoride is an extremely aggressive compound and its boiling point is relatively low (19,6oC) the conducting of physical and chemical studies engaging it is connected with great experimental difficulties. Electrochemical researches are obstructed due to absence of electrode materials, inert towards liquid anhydrous hydrogen fluoride. Hackerman, Snavely and Fiel had stidued the electrochemical behaviour of 23 anodes on anhydrous hydrogen fluoride with the additive 0,01 M NaF [4]. According to the authors’ opinion, only nickel, platinum and Monel metal out of studied materials proved to be perspective for using as anodes in anhydrous hydrogen fluoride. However, the corrosion rates of nickel and platinum are close in their value and therefore the application of platinum as anode is not efficient in terms of economy.
The work experience of electrochemists of our company in the field of electrochemical fluorination proved, that at anode polarization of Monel metal the dissolving of nickel from the anode surface occurred, copper layer remained on the surface, which at further polarization turned into non-conducting current copper fluoride layer and electrolysis stopped. Thus, the only applicable anode material for the processes of electrochemical fluorination is nickel. The present work is devoted to studying of anode behavior of nickel in liquid anhydrous hydrogen fluoride.
The measurements were conducted in the cell from polychlorotrifluoroethylene (Fluoroplast-3). Nickel wire, which, excepting operational surface, was covered with isolating film of Fluoroplast-3, was used as an electrode under research. Nickel electrode, which value of surface was no less, than 10 times more than the surface of electrode under research, was used as an auxillary electrode. Hydrogen electrode based on platinated platinum, described before [5], was the reference electrode. It was in the other fluoroplastic vessel and was separated from the studied one by the tap lined with fluoroplastic-4 and fluoroplastic capillary, which end was close to the surface of studied electrode.
During the studying of nickel anode stationary anode curves of charging in solution of anhydrous hydrogen fluoride with additives of n-tributylamine, water and potassium had been registered. The obtained dependencies of changing of electrode potential in time can be seen at Pic. 1.
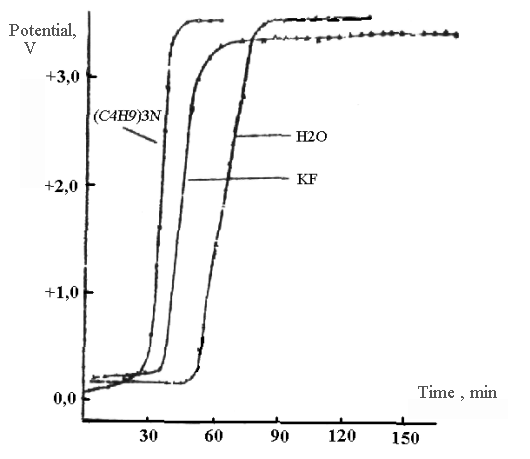
Pic. 1. Anode curve of charging on nickel in solutions of tributylamine, water and potassium fluoride.
The obtained curves are typical for for curves of passivating metals [6]. Judging by the picture we can make a conclusion, that curves lie in the field of practically the same potentials and are like each other. The lower part of the curves is in the field of low positive values and meets the active dissolution of nickel with formation of poorly soluble nickel fluoride. Its length depends on electrode curing time in solution, on preliminary polarization of electrode and density of polarizing current. Sharp growth of potential is observed at passivation. Potential corresponding to higher part of charging curves depends on density of polarizing current, but in all cases it lies above potential of reversible fluorine electrode. At present values of potential at the surface of electrode the conditions are being created at which the discharge of fluorine ions and further fluorination of dissolved compound, water or tributylamine can occur. The closeness of potentials of higher part of curves for water and tributylamine speaks in favour assumption made.
The conducted measurements proved, that nickel electrode was not inert in regards to solvent - liquid anhydrous hydrogen fluoride. Electrode’s surface is constantly changing as a result of forming or restoration of passivating layer.
It is clearly seen by the curves from Pic. 2 obtained at different conditions of keeping electrode without current and under cathode polarization.
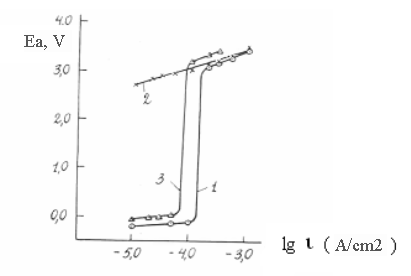
Pic.2. Anode polarization curves on nickel in solution 1m CH3COOH + 1m H2O: 1 – after preliminary cathode polarization; 2 – after obtaining curve 1; 3 – after 24 hours time after obtaining the curves 1 and 2 and preliminary cathode polarization
In order to define the influence of hydrocarbon radical length in fluorinated compound molecule the polarization curves in solutions of acetic CH3COOH and nonanoic C8H17COOH acids were obtained. The curves are listed at Pic. 3.
Besides that, the comparative experiments regarding electrochemical fluorination (ECF) of acetic CH3COOH and nonanoic C8H17COOH acids had been conducted at controlled anode’s potential. (Concentration of acids was equal to 5%, anode density of current was equal to 0,018 A/cm2). Anode’s potential in the electrolysis process changed within (4,2+0,2)V for acetic and (4,4+0,2)V for nonanoic acids.
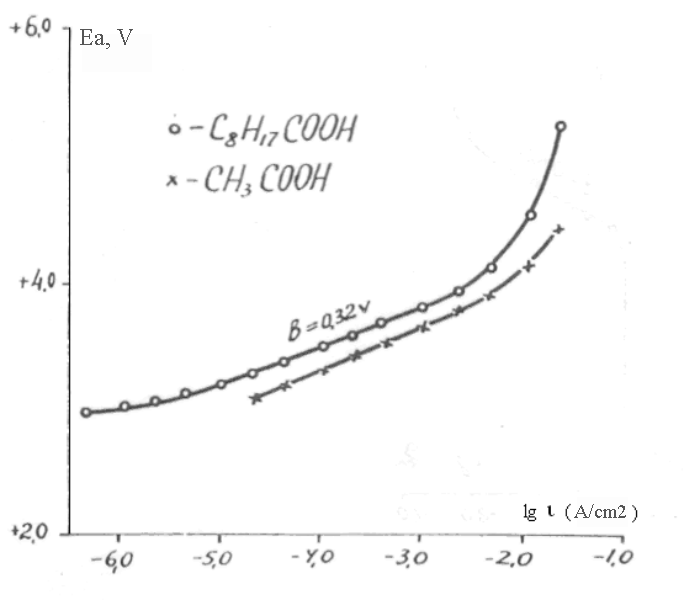
Pic. 3. Anode curves (reverse) on nickel in 5% solutions of CH3COOH and C8H17COOH
The obtained data conforms to the data of works of Hackerma [4] with co-workers and Vatanabe [7], in which for passive nickel in solutions of the same acids and also in solutions of 0,01M KF in HF with different additive of water the unusually high gradients of linear parts of polarization curves were obtained. Values of angle of inclination of curves in passive field were 0,40-0,62V for acids and 0,50-0,80V for KF+H2O. The authors also explain the obtained data by combination of a number of facts, including diffusion limitations of initial compounds at the surface of anode and due to that limitations by the growth of passive film as a result of reaction of fluorine radicals formed at discharge of fluorine-ions, with anode’s materials.
At Pic. 4 the curve of anode’s potential decline after turning off the polarizing current is listed. Sharp drop of potential from 5,29V occurred only at first minute after turning off the current. Then the potential changed very slowly. The extrapolation of this part of the curve up to the meaning of tmin=0 produces the meaning of potential of 2,82 V close to the potential of reversible fluorine electrode (2,87V according to Latimer).
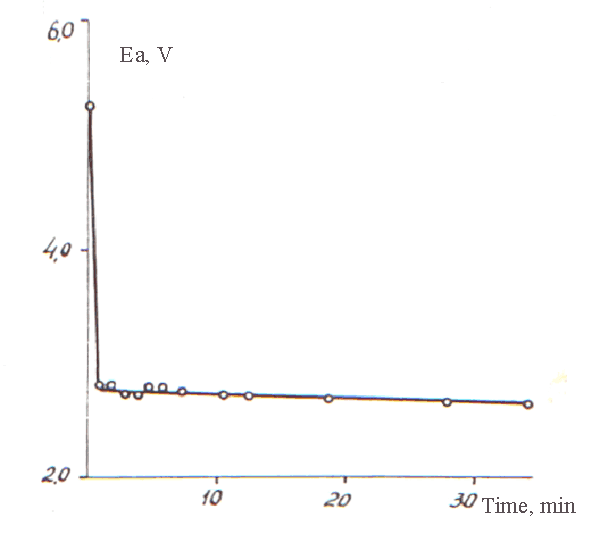
Pic. 4. Changing of Potential of Nickel Anode in Time After Turning the Current off (C8H17COOH)
In order to obtain more informative results the nickel passivation studies were continued by putting a quick galvanostatic impulse onto electrode, which had been kept at certain cathode potential.
Charging curves on nickel in pure anhydrous hydrogen fluoride and in solutions of acidic acid, water and pyridine had been registrated (Pic. 5).
Charging curves for pure anhydrous hydrogen fluoride and 0,05M CH3COOH were obtained at freshly cleaned electrode. Delays at low value of potential (0,1-0,5V depending on current density) connected with nickel dissolution and forming of nickel fluoride on its surface are observed at them as well as at the stationary charging curves.
Similar curves at cleaned electrode were obtained for pyridine and water as well. However, curves for pyridine and water listed at Pic. 5 had been obtained after multiply anode polarization of electrode without its preliminary cleaning. That’s why delays responding to the forming of passive nickel in present case are not seen. At the same time the fact, that except for pure anhydrous hydrogen fluoride at all curves we can observe the delay in the field of potentials about 2,2-2,5V, e.g. at potentials somewhat lower than the potential of reversible fluorine electrode drows our attention to it.
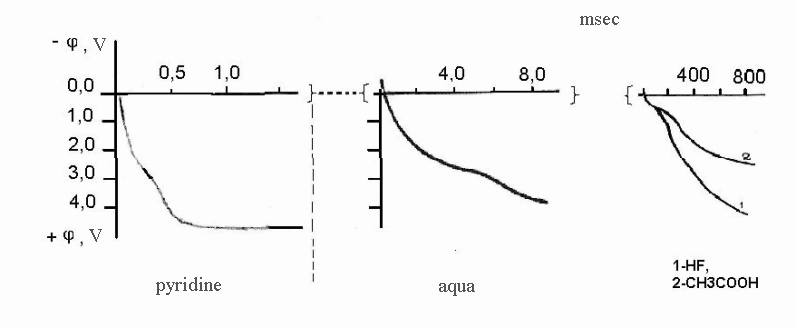
Pic. 5. Anode Charging Curves on Nickel (Quick Galvanostatic Impulse). Current Density: Pyridine and Water – 3x10-2A/cm2; acetic acid and HF – 1x10-2 A/cm2
The mentioned delay can’t be connected with the oxidation of compounds dissolved, as so different in structure and chemical activity compounds as water, acetic acid and pyridine can't have the same number of anode oxidation potential. It is absolutely natural to suppose, that obtained delay is connected with oxidation at anode of water, which admixture can be found in pyridine and acetic acid. Besides that, at dissolving of acetic acid in anhydrous hydrogen fluoride water is formed according to the following reaction:
|
CH3COOH+ HF |
------> CH3COF |
+ |
H2O |
(1) |
As the potential of this reaction is more negative than the potential of discharge of fluorine ions, then under these conditions fluorine can't take part in the process of water oxidation. The reaction of water oxidation at anode can be represented by the following summary equation:
|
2 H2O - 4e |
-----> |
O2 |
+ |
4H+ |
(2) |
We can suppose, that at present potentials the oxidation reaction of bivalent nickel to 3- or 4-valent nickel occurs according to the following equation:
|
NiF2 + F- - e |
-----> |
NiF3 |
(3) |
Or:
|
NiF2 +H2O + 3F- - e |
-----> |
NiF2O |
+ |
2HF |
(4) |
If reaction (3) had taken place, then the present delay would be observed in anhydrous hydrogen fluoride as well. Therefore the reactions (2) and (4) seem more likely to happen. The next delay observed at fast charging curves lies within potentials' field of more than 3V, e.g. at the potentials more positive than the potential of reversible fluorine electrode. This delay corresponds to the delay observed before at stationary charging curves. At dissolving of water and organic compounds in liquid anhydrous hydrogen fluoride the dissociation of HF takes place according to the following equation:
|
R + 2 HF |
------> |
RH+ |
+ |
HF2- , |
(5) |
where R=H2O, CH3COOH, C2H5N or (C4H9)3N.
Thus, at potentials corresponding to the last delay at charging curves the process of discharge of solvated ion of fluorine with formation of fluorine radical is the most probable electrochemical process:
|
HF2- - e |
-----> |
F. |
+ |
HF |
(6) |
The fluorination is carried out by fluorine radical adsorbed at passive nickel anode covered with the layer of nickel fluoride. As the only electrochemical process at potentials corresponding electrochemical fluorination is a discharge of fluorine ion and the rate of ECF process is determined by the initial compounds diffusion rate to the anode surface, then the showings of of ECF process will be determined by the structure of molecule of compound being fluorinated. The lower the sizes of molecule of the compound being fluorinated , the higher the (under the same equal conditions of electrolysis) cation RH+ diffusion rate to the anode surface will be (where according to equation (5) R - molecule of compound being fluorinated). A fact known from the practice of different compounds electrochemical fluorination speaks for that supposition, that at turning on the electrolysis at first the water is being fluorinated forming monooxides of fluorine and ozone according to the reaction:
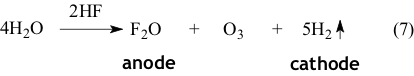
At that oxygen is being isolated at cathode. Thus, based on electrochemical measurements conducted it is proved, that fluorination of organic compound was carried out by fluorine radical formed as a result of fluorine ion discharge and adsorbed at passive nickel anode covered by the layer of nickel fluoride. It is proved, that the electrochemical fluorination process rate is defined by the diffusion limits near anode's surface. During the development of electrochemical fluorination technology and creation of production it is necessary to take into account the obtained data of kinetic studies of the process. That data showed, that the stage of diffusion of initial compounds to the anode surface and taking the reaction products away from it was the delayed stage of electrochemical fluorination process. This means, that the rate of electrochemical processes is defined by diffusion limits near the anode surface and the carrying out of the active mixing of electrolyte in the reaction zone, e.g. in field of electrode package is needed. The obtained data was used for modernization of electrolyzer to obtain perfluorinated dielectric liquids, in which the interior and exterior airlift are used for better mixing, forced circulation of electrolyte with application of pump with controlled mixing rate is introduced as well.
Conclusion
Based on conducted electrochemical studies of the electrochemical fluorination process it was proved, that in liquid anhydrous hydrogen fluoride the fluorination of organic compound was being carried out by fluorine radical formed as a result of fluorine ion discharge and adsorbed at passive nickel anode covered with the layer of nickel fluoride. It is stated, that electrochemical fluorination rate was defined by diffusion limits at anode surface and therefore active mixing of electrolyte in the reaction zone is needed in the process of electrochemical fluorination.
References
1. Saimons, G. H. Ftor i ego soedineniya / G. H. Saimons, H. Dzh. Emileus, A.B. Barg, V. Lange, G.S. Bus, D.T. Pinkston, D.R. Martin, D.H. Kedi, L.L. Bardger, G. Glokler, L.A. Bigelou, T.G. Brik, V. Pirlson, J. Pak, V.A. Veil. – M., IL, 1953. – T.1. – 509 s.
2. Odrit, L. Nevodnye rastvoriteli / L. Odrit, Ya. Kleinberg. – M., IL, 1955. – S.10; Rilpatrick, M. The Chemistry of Non-aqueous Solvent / M. Rilpatrick, J.G. Jones, Ed. J.J. Lagowski. – Acad. press, 1967. –V.2
3. Fredenhagen, K. / K. Fredenhagen, H. Fredenhagen // Z. anorgan. Und allgem. Chem. – 1939.– Vol. 243, № 39
4. Hackerman, N. / N. Hackerman, E.S. Snavely, L.D. Fiel // Corrosion Science. – 1967. – Vol. 7, № 39
5. Kaurova, G.I. Vodorodnyj elektrod sravneniya v srede zhidkogo ftoristogo vodoroda /Kaurova G.I., Grubina L.M., Adzhemyan TS.A. // Elektrohimiya. – 1967. – T. 3. – S. 1222
6. Fetter, K. Elektrohimicheskaya kinetika / K. Fetter, per. pod red.Kolotyrkina YA.M. – M., Khimiya, 1967. – S. 796-840
7. Chang, B. Electrochemical Fluorination of N-Nitrosodiethylamine / B. Chang, H. Yanase, K. Nakanishi and N. Watanabe // Electrohim. Acta. – 1971. – Vol. 16. – R. 1179; Watanabe, N. Electrochemical Fluorination of alkylamine / N. Watanabe, M. Haruta, B. Chang // Bull.Chem. Soc. Jpn. – 1972. – Vol. 45. – R. 1275
Fluorine Notes, 2010, 70, 5-6
