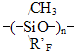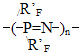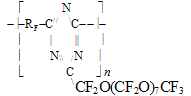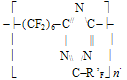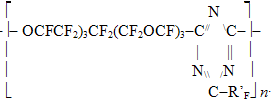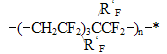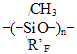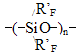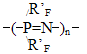Fluorine Notes, 2010, 69, 1-2
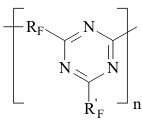
FORECASTING OF VITRIFICATION TEMPERATURE FOR FLUOROPOLYMERSA.N.Kollar, V.A.Gubanov Federal State Unitary Enterprise S.Y. Lebedev Research Institute for synthetic rubber (FSUE "NIISK") Abstract. Methods of calculating the Tg was tested on perfluoropolyoxaalkylene triazine polymers and used to calculate the Tg of carbochain copolymers of perfluoroalkyl vinyl ethers with vinylidene fluoride (tetrafluoroethylene). The methods of calculation will help to predict the properties of fluorinated polymers and Tg values for the new structures Keywords. Copolymers, fluoropolymers, perfluoroalkyl vinyl ethers, triazine It is well known that introduction of ether groups into the main or side chains of a polymer improves low-temperature properties of fluorelastomers and fluorocarbon oils [1]. Vitrification temperature (Tg) is known to be the major characteristic among low-temperature properties of amorphous polymers, particularly, elastomers; the said temperature is detected by various methods including those involved in the standard [2]. A number of methods proposed for the calculation of vitrification temperature [3, 4] were based on the polymer chemical structure reasoning from the idea that each constitutional repeating unit of the macromolecule under consideration should be divided into groups of atoms that contribute additive inputs into the equation for Tg independently of their surrounding. Those methods are not universal and their accuracy in fluoropolymer calculations never exceeded +/-10Co. The following equation for the calculation of (lg Tg) was proposed by A.A. Askadsky and G.L. Slonimsky [5] reasoning from the analysis of polymer chains packing factors: where: The similar approach was used to calculate Tg for fuorine-containing polymers, especially perfluoroalkyleneazines [7], through the introduction of Ki* increments corresponding to fluorine-containing groups. Numerical values of the related effective volumes were determined and vitrification temperatures were calculated for about 50 polymers. Unfortunately, at present there are virtually no methods for the production of high-molecule fluorocarbon polymers with ether bonds in their main chains, except for some polymers producible through the polycondensation, particularly perfluorooxaalkyltriazines. However, experimental Tg values for the most of such perfluoropolymers do not satisfy the said calculation schedule. In Table 1 there are Tg, values determined according to the state standard [2] for more than 40 perfluoropolyoxaalkylenetriazine polymers synthesized by us and structured as below:
RF - perfluoropolyoxaethylene- or perfluoropolyoxaisopropylene- unit in the main chain of a perfluoroalkylenetriazine polymer; R'F - perfluoro-3-oxapropyl-, perfluoro-1-methyl-2-oxapentyl- and perfluoro-1,4-dimethyl-2,5-dioxaoctyl, and also polyoxadifluoromethylene pendant in triazine ring. Table 1. Vitrification temperatures for perfluorotriazine polymers
The table analysis evidences that the introduction of fragments with ether oxygen into the main chain of a perfluorooxaalkylenetriazine polymer, especially due to tetrafluoroethyleneoxide units, decreases Tg considerably. In the case that oxygen is present in the chain due to hexafluoropropeneoxide units, the maximal effect of Tg decrease is achieved not only through the rise in number of oxygen-containing units in the main chain, but mostly through the rise in number of difluorooxamethylene units in side-chains R'F. This enables, when using perfluoropolyoxamethylene side chain groups in those polymers (in order to decrease Tg), to decrease the number of hexafluoromethylenoxide units between triazine rings. Therefore, one may conclude about multidirectional effects of oxygen-containing units in side and main chains, and that the maximal influence on the vitrification temperature is provided by oxygen of perfluoropolyoxamethylene groups in side pendants. In this connection in the equation for the calculation of vitrification temperature two different increment values are to be used for oxygen's in main and side chains, those being K*omain and K*oside . It is obvious that K*omain > K*oside. For each of those coefficients the effects of the number of diperfluoromethylene or methyl groups located in the oxygen α- and b-surroundings in Cn-O-Cm should be taken into account as an average: It is obvious that the effects of triazine ring on vitrification temperature should be taken into account by the introduction of a special coefficient K*tr. Reasoning from experimental Tg values for perfluoroalkylenetriazine polymers in table 1 and K*i,, used by A.A. Askadsky et al. [5, 6] the equation (1) was used to calculate the increment value K*oside for each oxygen in perfluoromethylene oxide units in side chains, K*1omain for each oxygen in the main polymer chain in hexafluoropropylene oxide units, K*2omain for oxygens in tetrafluoroethyleneoxide units and K*tr for triazine ring, all shown in table 2. Table 2. Novel incremental values in equation (1)
Using the novel increment values Tg were calculated for a number of perfluoroalkylenetriazine polymers, and thus obtained values are compared with experimental ones [2] in Table 3. Table 3. Calculated and experimental values of vitrification temperatures for perfluoroalkylenetriazine polymers
Root mean square deviation of the calculated value from that experimental does not exceed +/-5,2oC. To estimate the influence of oxygen in the main chain of hetero-chain co-polymers we, making use of K*onm increments calculated according to (2), estimated Tg values for a number of polyethers and compared them in Table 4 with experimental values found in literature [6-8]. It is obvious that the limit polyether vitrification temperature value will be that calculated for polydifluoromethylene oxide -170,5oC as shown in the table. Table 4. Calculated and experimental vitrification temperatures for perfluoropolyethers
* - to calculate Tgfor polyhexafluoropropyleneoxide with such structure K*2omain increment was applied. The method proposed for the calculation of vitrification temperature was tested with perfluoropolyoxaalkylenetriazine polymers and used to calculate Tg values for carbon-chain co-polymers of perfluoroalkylvinyl ethers with vinylidenefluoride and tetrafluoroethylene produced through emulsion polymerization. In Table 5 thus calculated values are compared with those experimental. Table 5. Calculated and experimental vitrification temperatures for carbon-chain fluoropolymers
*for hydrogen-containing polymers the effect of hydrogen bond increment was taken into account in our calculations. Root mean square deviation of the calculated values from the experimental ones does not exceed +/-6,5oC. In the calculations of Tg for the structure - [(- CÍ2CF2)3CF(R'F)CF2- )]n- typical for many fluoroelastomers with ratio 75:25 % mol. vinylidenefluoride and rubber-like co-monomer, such as hexafluoropropylene or various perfluoroalkyl- oxaalkylvinyl ethers, one may apply the empirical formulae based on equations (1, 2): Vitrification temperatures were calculated for the most frostproof heterochain fluoropolymers of various nature, particularly polyfluorosiloxanes and polyfluorophosphazenes. Table 6. Calculated and experimental vitrification temperatures for some hetero-chain fluoropolymers
Root mean square deviation of the calculated value from the experimental one does not exceed +/-6oC?. To calculate Tg for fluorophosphazene polymers one may apply the empirical equation as follows: Thus developed methods for the calculation of Tg values make it possible to forecast the properties of certain fluoropolymers, and forecast Tg values for some novel promising structures. Thus calculated values are shown in Table 7. Table 7. Calculated Tg values for triazine structures
Therefore, reasoning from the mentioned experimental and calculated results one may conclude that the pattern of calculation proposed for vitrification temperature makes it possible to forecast rather satisfactorily freezeproof characteristics of various carbon-chain and heterochain fluoropolymers in order to select the promising materials with the optimal properties. Apparently, among the investigated materials polyfluorooxamethylenephosphazene polymers have the best freeze resistance, as for them Tgvalues are close to its limit value - 170oC. List of References [1] Sinteticheskij kauchuk/ Pod red. I.V.Garmonova. L.: Khimiya, 1983. 560 s. [2] GOST 12254-66. [3] Van Krevelen D.B. Svojstva i khimicheskoe stroenie polimerov: Pod red. A.Ya.Mapkina. M.: Khimiya, 1976. 416 s. [4] Becher R. // Faserforschung und Textiltechnik Zeitschrift fur Polymerforsch, 1978. V. 29. N 6. P. 361-385. [5] Askadskij A.A.‚ Slonimskij G.L. // VMS. 1971. T. 14(A). N 8. C. 1917 - 1919. [6] Yarosh A.A.‚ Askadskij A.A.‚ Krukovskij S.P. i dr. // VMS. 1974. T. 16(A). N 3. C. 527-542. [7] Rice D.E. // J. Polymer Sci., Part A: Polymer Letters.1968. V. 6, N 5. P. 335 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fluorine Notes, 2010, 69, 1-2
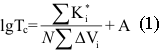
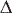 T,
T,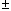 oC
oC