Fluorine Notes, 2010, 68, 3-4
New Obtaining Method For Polyfluorinated β-DiiminesM.A. Kurikin, D.Yu.Vebritsky The establishment of Russian Academy of Sciences
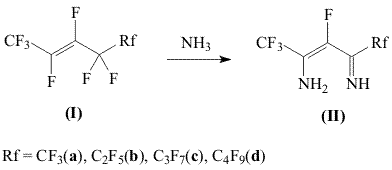
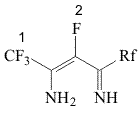
A.N.Nesmeyanov Institute of Organoelement Compounds RAS, Aliphatic polyfluorinated β-diimines (II), firstly described in 1980 [1], appeared to be a class of compounds with prospects. Polyfluorinated β-diketones (heptafluoroacetylacetone and its homologes) [2], different heterocyclic compounds - pyridines and pyrimidines, supporting fluoroalkyl substituents [3,4,5] were obtained based on them. Besides that, polyfluorinated β-diimines (II), being bis-azaanalogues of β-diketones are ligands of a new type. The present article is devoted to the development of a new laboratory obtaining method for polyfluorinated β-diimines (II). All of the β-diimines (II) synthesis methods offered before and the one discussed in this article are based on the interaction of linear perfluoroalknenes-2 (I) and ammonia: In the first of the described methods [1] the reaction of alkenes (I) and ammonia was carried out in autoclave. Natural limitations, which are caused by the size of autoclave, are the are the drawbacks of the present method. The synthesis method (II) using the interaction (I) with aqueous ammonia which was offered later [2] doesn't lack in drawbacks as well. Olefines (I) do not mix with aqueous ammonia and higher volumes of solvents, which are inevitably lost at processing the reaction mass with water, are needed to carry out the reaction. Method offered here is based on the interaction of alkenes (I) and aqueous ammonia in the medium of ether at temperatures of -40oC : -30oC. The present method has got significant advantages compare to the ones described before [1,2]: the reaction is carried out at atmospheric pressure, it is easily enlarged to the multilitre reactors, the solvent used is returned into the cycle with minimum losses. Experimental Part NMR 1H and 19F spectra are recorded using the "Bruker AV-300" (300 and 282,38 MHz) spectrometer, chemical shifts are listed in ppm scale is relative to CF3COOH and TMS , spin-spin coupling in Hz. 1200 ml of diethyl ether were put into the glass reactor equipped with drop funnel, reflux condenser cooled by the mixture of dry ice-acetone, and thermometer. Ether were cooled to -42oC and 200 ml of liquid ammonia were poured into the reactor. Then at constant mixing (magnetic mixer) 117.1 g (0.47 mole) of perfluoropentene-2 (Ia) were added to the mixture obtained. The reaction mass was being mixed for 30 minutes, then the cooling was off and the solution was let to warm up at room temperature. The reaction mixture of orange color with white residue was poured into water, the ether layer was separated , washed with water (3X1000 ml), dried over CaCl2. Ether was distilled, 75.8 g (72%) 2-amino-4-iminoperfluoropentene-2 (II a) were obtained by the distillation. Other β-diimines (II b-d) were synthesized by analogy; spectral data and physical constants of obtained compounds are listed Table 1. Table 1. Spectral Characteristics and Physical Constants of Obtained Compounds
References 1. M.A. Kurykin, L.S. German, I.L. Knunyants, Izv. AN SSSR, Ser. Khim, 1980, 12, 2827. 2. O.E. Petrova, M.A. Kurykin, D.V. Gorlov, Izv. AN SSSR, Ser. Khim, 1999, 9, 1710. 3. O.E. Petrova, M.A. Kurykin, E.M. Kagramanova, Izv. AN SSSR, Ser. Khim, 2002, 4, 656. 4. O.E. Petrova, M.A. Kurykin, E.I. Mysov, Khimiya geterotsiklicheskih soedinenij, 2004, N 8, 1184. 5. D.Yu. Verbitskij, M.A Kurykin, J. Fluorine Notes, 2009, 3(64). |
Fluorine Notes, 2010, 68, 3-4