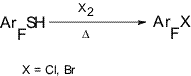Fluorine Notes, 2010, 68, 1-2
SUBSTITUTION OF THIOL GROUPS IN POLYFLUOROARENETHIOLS FOR CHLORINE AND BROMINE ATOMS. PRODUCTION OF CHLORO- AND BROMOPOLYFLUOROARENESV.E.Platonov, P.V.Nikulshin, A.M.Maximov Novosibirsk N.N. Vorozhtsov Institute of Organic
Chemistry of SB RAS 630090, Novosibirsk, prospect Acad. Lavrentiev, 9
Chlorine-containing and bromine-containing polyfluoroarenes are major raw materials used in the production of a broad spectrum of polyfluoroaromatic substances with various functional groups [1]. Yet, only the most simple chloro- and bromopolyfluorobenzenes are rather easily accessible (chloro- and bromopentafluorobenzene, dibromotetrafluorobenzenes). It is well known that chloropentafluorobenzene (1) is formed (with yield 20%), along with hexafluorobenzene (21%), and polychlorofluorobenzenes, when hexachlorobenzene is heated with potassium fluoride at 450-500oC [2]. In order to produce (1) it also was proposed to chlorinate pentafluorobenzene with the help of Cl2 [3], HCl or NaCl [4] in the presence of SbF5 or using a complex of chlorofluorosulfate with SbF5 [5]. Through the action of bromine on pentafluorobenzene in 65% oleum [6] or in 20% oleum in the presence of AlBr3 bromopentafluorobenzene (2) was produced (yield 81%) [7]. Under similar conditions tetrafluorobenzenes are brominated to their dibromo-derivatives (yield 38-78%) [8, 9]. However, the attempts to chlorinate 1,2,4,5-tetrafluorobenzene through the action of SO2Cl2 in 65% oleum did not meet success [10]. Having in mind to develop a general method for the production of chloro- and bromopolyfluoroarenes and their heteroanalogues, we investigated the possibilities for easy and sufficiently selective nucleophilic substitution of thiol groups in polyfluoroarenes [11] for chlorines or bromines. We have shown that during the co-pyrolysis of polyfluoroarenethiols with chlorine or bromine in a flow-through system at 300-650oC the substitution of thiol groups for chlorines or bromines occurs resulting in the formation of related halogen-containing polyfluoroarenes (diagram 1) [12].
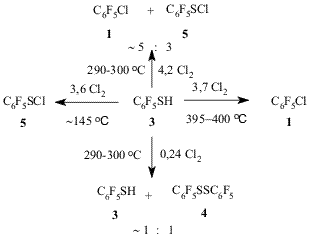
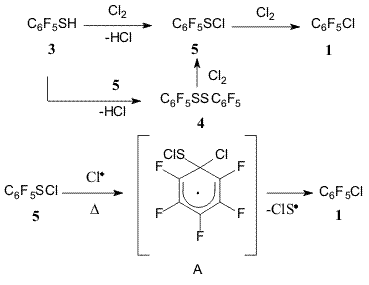
Diagram 1 The method provides with good yields a simple route to a wide spectrum of various chloro- and bromopolyfluoroarenes. While so doing the process occurs with high selectivity making it possible to synthesize individual isomeric substances. The co-pyrolysis of pentafluorobenzenethiol (3) with chlorine or bromine results in the formation of substances (1) or (2). Decafluorodiphenyldisulphide (4) and pentafluorobenzenesulphenylchloride (5) also undergo similar conversions under the action of Cl2 (diagram 2) [12]. Diagram 2 At lower temperatures ~145oC the reaction between thiol (3)
and Cl2 results in high yield of sulphenylchloride (5)
with only small admixture of substance (1), while at 290-300oC
and decreased chlorine consumption ((3):Cl2 ~ 4:1) the
reaction mixture contains non-reacted thiol (3) and disulphide (4),
their mole ratio being ~1:1, and trace amounts of substance (1). If
at 290-300oC the mole ratio of thiol (3) to Cl2 is ~ 2:1 the process results in substances (3), (4) and (5)
produced with mole ratio ~1:4:17 [12]. The further rise in Cl2 consumption ((3):Cl2 ~ 1:4) brings about to formation a
blend of substance (1) (major product) and sulphenylchloride (5)
((1):(5) ~ 5:3) (diagram 3) [12].
Diagram 3 Judging by the data of chromato-mass-spectrometry the pyrolysis of sulphenylchloride (5) (at 395oC) results in the formation of substance (1) and disulphide (4), and, to a lesser extent, in that of decafluorodiphenylsulphide and decafluorodiphenyltrisulphide [12]. Recently the formation of substance (1) with quantitative elimination of elemental sulfur from substance (5) at 700-750oC (10-13 Pa, 3.5 h) was reported [13]. At 405-410oC disulphide (4) reacts with Cl2 to give substance (1) with high yield [12]. The maximal yield of substance (2) in co-pyrolysis of thiol (3) with Br2 (1:2,7) is achieved at temperatures higher (500oC) than that of substance (1) formation when thiol (3) reacts with Cl2 (400oC). Either drop or rise in the reaction temperature results in lower yields of the target product in the reaction between thiol (3) and Br2. The yield of substance (1) decreases with the amount of Br2 taken for the reaction. Disulphide (4) and Br2 at 500oC produce substance (2) as well [12]. Reasoning from the above data on the co-pyrolysis of thiol (3) with Cl2 at various conditions a mechanism of the substance (1) formation was suggested that involved initial conversion of thiol (3) under the action of Cl2 to give sulphenylchloride (5). The latter reacts with Cl2 to form substance (1). Sulphenylchloride (5) in the presence of thiol (3) may form disulphide (4) [14], the latter is then converted to substance (1) under the action of Cl2. According to [15] at first disulphide (4) reacts with Cl2 giving sulphenylchloride (5). The latter therefore may perform as an intermediate product on the way from thiol (3) and disulphide (4) to substance (1) (diagram 4). However, one must not rule out the possibility of direct substitution of HS or C6F5SH groups for chlorines in substances (3) and (4). Diagram 4
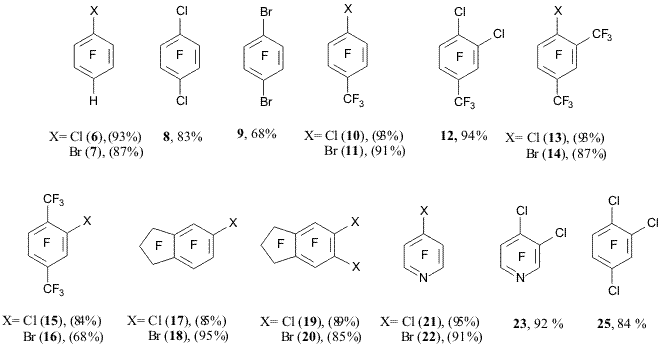
The substitution of RS groups by chlorines are not in conflict with the data on C-X binding energies in C6H5X molecules, here X = SH (355.5 kJ/mole), F (510.8 kJ/mole), Cl (396.9 kJ/mole), H (457.6 kJ/mole), CF3 (448.8 kJ/mole) [17], C-S bond being the less strong. The results of the comparison with C-Br binding energies (328.7 kJ/mole) in C6H5Br are not so obvious [17]. It is conceivable that in the formation of polyfluorobromoarenes the substitution of BrS (and ArFSH) groups for bromines acts its part, if we assume downtrend in CArF-SX (X = Br, S-ArF) binding energy depending on the nature of X as to compare with the binding energy of ArF-SH. Thus, when passing from benzenethiol to methylthiobenzene and diphenylsulphide the CAr-S binding energy tends to go down [17]. The co-pyrolysis of 2,3,5,6-tetrafluorobenzenethiol with Cl2 or Br2 occurs smoothly resulting in 1 chloro-2,3,5,6-tetrafluorobenzene (6) and 1-bromo-2,3,5,6-tetrafluorobenzene (7) (diagram 5). When so doing, the replacement of hydrogens by halogens in the ring was never observed in fact [12]. Similar process has place as well for other thiol (3) derivatives that contain chlorines, bromines or trifluoromethyl groups in para-position in their tetrafluorobenzene rings. The co-pyrolysis of 4 chlorotetrafluorobenzenethiol with chlorine at 400oC resulted in 1,4 dichlorotetrafluorobenzene (8), and that of 4 bromotetrafluorobenzenethiol with bromine at 500oC gave 1,4-dibromotetrafluorobenzene (9). Both p-chloro- and p-bromoheptafluorotoluenes (10) and (11) were also synthesized from 4 trifluoromethyl 2,3,5,6 tetrafluorobenzenethiol (diagram 5) [12]. In the reaction of 4-trifluoromethyl-3,5,6-trifluoro-2-chlorobenzenethiol with Cl2 (400 oC) the yield of 1,2 dichloro-4-trifluoromethyl-3,5,6-trifluorobenzene (12) was 94% (diagram 5). It was shown that the co-pyrolysis of 2,4-bis(trifluoromethyl)-3,5,6-trifluorobenzenethiol with Cl2 at 400oC or with Br2 at 500oC results in 1-chloro-2,4-bis(trifluoromethyl)-3,5,6-trifluorobenzene (13) [18] and 1-bromo-2,4-bis(trifluoromethyl)-3,5,6-trifluorobenzene (14) relatively (diagram 5) [12]. In the similar manner 2,5-bis(trifluoromethyl)-3,4,6-trifluorobenzenethiol was used in the synthesis of 1-chloro-2,5-bis(trifluoromethyl)-3,4,6-trifluorobenzene (15) [18] and 1-bromo-2,5 bis(trifluoromethyl)-3,4,6-trifluorobenzene (16) (diagram 5) [12]. The presence of CF3 group in orto-position to SH-group does not rule out its substitution for chlorines or bromines. Co-pyrolysis of 5-nonafluoroindanthiol with Cl2 (at 400oC) or with Br2 (at 500oC) results in 5-chlorononafluoroindan (17) [18] and in 5-bromononafluoroindan (18) accordingly (diagram 5) [12]. Through the action of KSH substances (17) and (18) are converted to 6-chlorooctafluoroindan-5-thiol and 6-bromo-octafluoroindan-5-thiol, those two react with Cl2 (400oC) or Br2 (500oC) giving 5,6-dichloro-octafluoroindan (19) and 5,6-dibromo-octafluoroindan (20) [19] relatively (diagram 5). This type of hetarene conversion is exemplified by the reaction of 4-tetrafluoropyridothiol with Cl2 (400oC) or Br2 (500oC) resulting in the formation of 4-chlorotetrafluoropyridine (21) and 4-bromotetrafluoropyridine (22) respectively [12] (diagram 5). 3-chlorotrifluoropyrido-4 thiol is also smoothly chlorinated at 400oC resulting in 3,4-dichloro-2,5,6 trifluoropyridine (23) (diagram 5). It was shown that 2,5-dichloro-3,4,6-trifluorobenzenethiol (24) reacts with Cl2 (400oC) to form 1,2,4- trifluorotrichlorobenzene (25) (diagram 5) [20]. Thiol (24) was synthesized from substance (8) through the action of KSH [20]. Substance (8) being not easily available from technical standpoint, a more practicable method for the product (25) manufacture was developed. The method makes use of easily available technical-grade mixture of isomeric dichlorotetrafluorobenzenes that is produced through the reaction of hexachlorobenzene with KF. The mixture consists mainly of m-dichlorotetrafluorobenzene besides of o- and p-isomers [2]. By the action of KSH on the dichlorotetrafluorobenzene mixture (with ratio m: o: p = 44: 10: 10) 2,4-, 2,5- and 3,4-dichlorotrifluorobenzenethiols were produced, their ratio being ~43: 10: 10, judging by their NMR19F spectra. The latter is close to the initial ratio of isomeric dichlorotetrafluorobenzenes. Orientation of m- and o-dichlorotetrafluorobenzenes in their reaction with KSH is in good conformity with literature data on those substances nucleophilic substitution when reacted with sodium methylate [21]. The co-pyrolysis of thus produced dichlorotrifluorobenzenethiol mixture with Cl2 at 400oC results in 1,2,4-trifluorotrichlorobenzene (25) with good yield (diagram 5) [20]. Diagram 5 Therefore, various chloro- and bromopolyfluoroarenes were produced through the co-pyrolysis of polyfluoroarenethiols with chlorine and bromine. A number of novel chlorine- or bromine-containing derivatives of perfluoroarenes are synthesized. Some of thus produced substances are practically inaccessible when using any other methods. It also should be marked that the method developed by us is well applicable in the synthesis of hardly accessible o-dichloroderivatives of perfluoroarenes and trifluorotrichlorobenzene (25). References 1. Sintezy ftororganicheskih soedinenij. Red. Knunyants I.L., Yakobson G.G. - M. Khimiya, 1973, 108. 2. Vorozhtsov-jr. N.N., Platonov V.E., Yakobson G.G. Poluchenie geksaftorbenzola iz geksahlorbenzola // Izv. AN SSSR. Ser. khim. 1963, 1524. 3. Dobronravov P.N., Shteingarts V.D. Ftorsoderzhashchie karbokationy. XV. Elektrofil'noe chlorirovanie pentaftorbenzola i geksaftornaftalinov. Arenonievyj ion, otvechayushchij zameshcheniyu vodoroda chlorom v 1-N- geptaftornaftaline // ZhOrKh. 1977, 13, 1679. 4. Brovko V.V., Sokolenko V.A., Yakobson G.G. Aromaticheskie ftorproizvodnye. LIV. Alkilirovanie pentaftorbenzola 1,1,2-trihlortriftoretanom i ftoroformom v prisutstvii pyatiftoristoj sur'my // ZhOrKh. 1974, 10, 300. 5. Fokin A.V., Studnev Yu.N., Krotovich I.N., Furin G.G., Yakobson G.G. Vzaimodejstvie poliftoraromaticheskih soedinenij s kompleksom ftorsul'fata chlora s pyatiftoristoj sur'moj // Izv. AN SSSR. Ser. khim. 1981, 927. 6. Pummer W.J., Wall L.A. Reactions of pentafluorohalobenzenes // J. Res. Nat. Bur. Stand. 1959, 63a, 167. 7. Nield E., Stephens R. Tatlow J.S. Aromatic polyfluoro-compounds. Part I. The synthesis of aromatic polyfluoro-compounds from pentafluorobenzene // J. Chem. Soc. 1959, 166. 8. Nield E., Stephens R., Tatlow J. C. Fluorocyclohexanes. Part VI. Some hexa- and pentafluorocyclohexenes and their dehydrofluorination // J. Chem. Soc. 1960, 3800. 9. Hellmann M., Bilbo A. J. The preparation of two fluorinated p-dihalobenzenez // J. Am. Chem. Soc. 1953, 75, 4590. 10. Hellmann M., Bilbo A.J., Pummer W. J. Synthesis and properties of fluorinated polyphenyls // J. Am. Chem. Soc. 1955, 77, 3650. 11. Brooke G.M. The preparation and properties of polyfluoro aromatic and heteroaromatic compounds // J. Fluor. Chem. 1997, 86, 1. 12. Platonov V.E., Maksimov A.M.,.Dvornikova K.V, Nikul'shin P.V. Ftororganicheskie serosoderzhashchie soedineniya. V. Sopiroliz poliftorarentiolov, -getarentiolov i ih proizvodnyh s hlorom i bromom // ZhOrKh. 2005, 41, 1681. 13. Sartori P., Golloch A. Notiz zur chemie des pentafluorbenzolsulfenylchlorids // Chem. Ber. 1970, 103, 3936. 14. Neil R.J., Peach M.E. Some reactions of pentafluorobenzenesulfenyl chloride and pentafluorobenzenesulfenyl cyanate // J. Fluor. Chem. 1971/72, 1. 257. 15. Robson P., Smith T.A., Stephens R., Tatlow J.C. Aromatic polyfluorocompounds. Part XIII. Derivatives of penta- and 2,3,5,6-tetra-fluorothiophenol // J. Chem. Soc. 1963, 3692. 16. Neil R.J., Peach M.E., Spinney H.G. Pentafluorobenzenesulfenyl halides and pseudohalides // Inorg. Nucl. Chem. Lett. 1970, 6, 509. 17. Energii razryva khimicheskih svyazej. Potentsialy ionizatsii i srodstvo k elektronu. Red. Kondrat'ev V.N., M. Nauka. 1974, 63. 18. Platonov V.E., Maksimov A.M., Nikul'shin P.V. Chlor- i brompoliftorareny. Razrabotka metoda polucheniya na osnove poliftorarentiolov // Konferentsiya RFFI «Fundamental'naya nauka v interesah razvitiya kriticheskih tekhnologij» s mezhdunarodnym uchastiem, Vladimir (Russia). 2005. Tezisy dokladov, 56. 19. Platonov V.E., Maksimov A.M., Nikul’shin P.V. Synthesis and thermolytic reactions of polyfluoroarenethiols // 13. Deutscher Fluortag and 7th Regular German-Russian-Ukrainian Symposium on Fluorine Chemistry. Schmitten (Germany). Sept. 29 – Oct. 2, 2008. 20. Platonov V.E., Maksimov A.M., Nikul'shin P.V. Sintezy 1,2,4-trihlortriftorbenzola, 2,4,5-trihlor-3,6-diftorfenola i –tiofenola – bazovyh soedinenij dlya sozdaniya novyh ftorsoderzhashchih pestitsidov // II konferentsiya «Fundamental'naya nauka v interesah razvitiya khimicheskoj i himiko- farmatsevticheskoj promyshlennosti», Perm' (Rossiya). 2004. Tezisy dokladov, 209. 21. Chambers R.D., Close D., Lyn D., Williams H. Mechanisms for reactions of halogenated compounds. Part 3. Variation in activating influence of halogen substituents in nucleophilic aromatic substitution // J.C.S. Perkin II. 1980, 778. |
Fluorine Notes, 2010, 68, 1-2